¶ Hydrogen Production by IS process using a High-Temperature Gas-cooled Reactor
¶ 1. Roadmap Overview
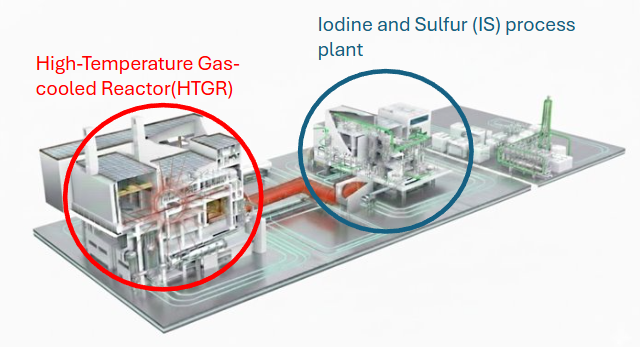
The High-Temperature Gas-cooled Reactor (HTGR) is an advanced reactor characterized by its ability to extract heat at extremely high temperatures ranging from 700°C to 950°C. The fuel is multi-layered with heat-resistant ceramic materials, the internal structures use graphite with high thermal resistance, and helium which undergoes minimal phase changes or chemical reactions even at high temperatures is used as the coolant. This enables operation at significantly higher temperatures compared to light water reactors. This temperature range is essential for driving an efficient thermochemical hydrogen production cycle.
The IS process produces hydrogen by using chemical reactions of iodine (I) and sulfur (S) driven by a heat source up to 900 °C to thermally decompose water. By combining three chemical reactions, the required temperature for water decomposition can be reduced from about 4000 °C, the temperature needed for direct thermal decomposition, to below 900 °C.
Moreover, because iodine and sulfur circulate within the process, only hydrogen and oxygen are produced from water, allowing CO₂-free hydrogen production.
For the goal of 2050 carbon neutrality, the decarbonization in the industrial sector, especially in the steel and chemical sectors, is crucial, as these sectors account for about 25% of Japan’s total CO₂ emissions. Achieving the ambitious goal will require a large-scale and low-cost supply of hydrogen.
Various methods for hydrogen production have been proposed, ranging from demonstration to practical use. Methods that take advantage of high temperatures to improve efficiency include methane steam reforming, high-temperature steam electrolysis, methane thermal decomposition, and the thermochemical IS process developed by the Japan Atomic Energy Agency (JAEA).
¶ 2. Design Structure Matrix (DSM) Allocation
The roadmap tree for hydrogen production shows that it can be realized through two main systems: the High-Temperature Gas-cooled Reactor and the Hydrogen Production System. Each system decomposes into subcomponents such as the reactor, heat exchanger, and turbine, which in turn require enabling technologies and materials at level 5, including nuclear fuel, helium gas, and chemical reactants (I₂, SO₂, H₂O, etc.).
Below is the DSM diagram for HTGR-IS Process Hydrogen Production.
¶ 3. Roadmap Model using OPM
The OPM (OPD & OPL) for HTGR-IS Process Hydrogen Production is as follows. The system configuration, which connects heat generation by the HTGR with the chemical reactions of the IS process, is illustrated in this diagram. The FOMs are also represented on the OPM.
OPL for HTGR-IS Process Hydrogen Production is displayed below.
High-temperature Gas-cooled Reactor is a physical and systemic object.
Reactor is a physical and systemic object.
Intermediate Heat Exchanger is a physical and systemic object.
Gas Turbine is a physical and systemic object.
Nuclear Fuel is a physical and systemic object.
Uranium is a physical and systemic object.
Ceramics is a physical and systemic object.
He Gas is a physical and systemic object.
900℃ Temperature is a physical and systemic object.
I2 is a physical and systemic object.
SO2 is a physical and systemic object.
H2O is a physical and systemic object.
HI is a physical and systemic object.
H2SO4 is a physical and systemic object.
O2 is a physical and systemic object.
H2 is a physical and systemic object.
400℃ Temperature is a physical and systemic object.
Hydrogen Production System is a physical and systemic object.
Bunsen Reactor is a physical and systemic object.
Sulfuric Acid Decomposition Reacto is a physical and systemic object.
HI Decomposition Reactor is a physical and systemic object.
FoM is a physical and systemic object.
Capital Cost of High-temperature Gas-cooled Reactor is an informatical and systemic object.
Availability Factor of High-temperature Gas-cooled Reactor is an informatical and systemic object.
Core Outlet Temperature of Generating is an informatical and systemic object.
Thermal Output of Generating is an informatical and systemic object.
Power Density of Generating is an informatical and systemic object.
Thermal Efficiency of Generating is an informatical and systemic object.
Levelized Cost Of Energy of Generating is an informatical and systemic object.
Fuel Burnup of Generating is an informatical and systemic object.
Hydrogen Production Rate of Producing is an informatical and systemic object.
Heat-to-hydrogen Efficiency of Producing is an informatical and systemic object.
Hydrogen Production Cost of Producing is an informatical and systemic object.
Co₂ Emissions of Producing is an informatical and systemic object.
Is Process Thermal Efficiency of Producing is an informatical and systemic object.
System Coupling Efficiency of Producing is an informatical and systemic object.
Chemical Recycle Rate of Producing is an informatical and systemic object.
Sulfuric Acid Decomposition Temperature of Transforming is an informatical and systemic object.
High-temperature Gas-cooled Reactor consists of Gas Turbine,Intermediate Heat Exchanger, and Reactor.
Nuclear Fuel consists of Ceramics and Uranium.
Hydrogen Production System consists of Bunsen Reactor,HI Decomposition Reactor, and Sulfuric Acid Decomposition Reacto.
Availability Factor,Capital Cost,Chemical Recycle Rate,Core Outlet Temperature,Co₂ Emissions,Fuel Burnup,Heat-to-hydrogen Efficiency,Hydrogen Production Cost,Hydrogen Production Rate,Is Process Thermal Efficiency,Levelized Cost Of Energy,Power Density,Sulfuric Acid Decomposition Temperature,System Coupling Efficiency,Thermal Efficiency, and Thermal Output are FoMS.
High-temperature Gas-cooled Reactor exhibits Availability Factor and Capital Cost.
Generating exhibits Core Outlet Temperature,Fuel Burnup,Levelized Cost Of Energy,Power Density,Thermal Efficiency, and Thermal Output.
Producing exhibits Chemical Recycle Rate,Co₂ Emissions,Heat-to-hydrogen Efficiency,Hydrogen Production Cost,Hydrogen Production Rate,Is Process Thermal Efficiency, and System Coupling Efficiency.
Transforming exhibits Sulfuric Acid Decomposition Temperature.
Generating is a physical and systemic process.
Generating requires He Gas,Nuclear Fuel, and Reactor.
Generating yields 400℃ Temperature and 900℃ Temperature.
Transforming is an informatical and systemic process.
Transforming requires Bunsen Reactor.
Transforming consumes H2O and SO2.
Transforming yields H2SO4.
Producing is a physical and systemic process.
Producing requires 900℃ Temperature and Sulfuric Acid Decomposition Reacto.
Producing consumes H2SO4.
Producing yields O2 and SO2.
Producing is a physical and systemic process.
Producing requires 400℃ Temperature and HI Decomposition Reactor.
Producing consumes HI.
Producing yields H2 and I2.
Transforming is an informatical and systemic process.
Transforming requires Bunsen Reactor.
Transforming consumes H2O and I2.
Transforming yields HI.
(ISO 19450)
¶ 4. Figures of Merit (FOM)
This project is an integrated system combining two complex technological fields: the High-Temperature Gas-cooled Reactor (HTGR) as the heat source and the Iodine-Sulfur (IS) Process as the hydrogen production plant.
Therefore, to comprehensively evaluate the feasibility and competitiveness of the system, a set of comprehensive Figures of Merit (FOMs) is required, covering both the performance and safety of the reactor unit, and the efficiency and cost of the chemical process unit. The key FOMs specific to the HTGR and IS process technological fields are listed below.
Table 4.1 High-Temperature Gas-cooled Reactor (HTGR) FOMs
|
FOM |
Units |
Description |
| Thermal Output | MWth | The total thermal energy generated by the reactor. |
| Core Outlet Temperature | ℃ | The temperature of the helium coolant as it exits the reactor core. A key performance indicator for HTGRs |
| Thermal Efficiency | % | The percentage of the reactor's thermal energy that is converted into a usable form, such as electricity. |
| Power Density | MW/m³ | he thermal output per unit volume of the reactor core. |
| Fuel Burnup | MW/t | The amount of energy extracted from a metric ton of nuclear fuel. |
| Capital Cost | USD/MW | The cost of construction per kilowatt of electrical output capacity. |
| Levelized Cost of Energy (LCOE) | USD/MWh | The average total cost to build and operate the plant over its lifetime divided by its total energy output. |
| Availability Factor | % | The percentage of time the reactor is operational over a given period. |
Table 4.2 HTGR-IS Process Hydrogen Production FOMs
|
FOM |
Units |
Description |
| Hydrogen Production Rate | NL/h | The volume or mass of hydrogen produced per hour. |
| Hydrogen Production Efficiency | % | The percentage of the HTGR's thermal energy that is converted into the chemical energy of the produced hydrogen. |
| Hydrogen Production Cost | USD/kg-H2 | The total cost to produce one kilogram of hydrogen. |
| CO₂ Emissions | kg -CO₂/kg -H₂ | he thermal output per unit volume of the reactor core. |
| IS Process Thermal Efficiency | % | The efficiency of the chemical process itself, defined as the energy of the produced hydrogen divided by the thermal energy supplied to the process. |
| System Coupling Efficiency | % | A measure of how effectively heat is transferred from the HTGR's helium coolant to the IS chemical plant. |
| Sulfuric Acid Decomposition Temperature | °C | The temperature required for the highest-temperature reaction in the IS cycle. |
| Chemical Recycle Rate | % | The efficiency with which the iodine and sulfur compounds are separated and recycled within the process loop. |
While only a few data points are available, a series of data points and its trendline show that HPR has improved exponentially. This trend suggests the technology has entered the Take-off phase in its lifecycle. However, it is important to note that HPR is an extensive metric proportional to the thermal output (scale) of the facility. The increase in HPR primarily demonstrates the successful continuous scale-up of the test facility size (transition to commercial realization), rather than a pure improvement in the intrinsic efficiency of the process technology (intensive progress). Therefore, the true significance of this HPR trend lies in its strong suggestion that technical barriers to large-scale, stable hydrogen supply at industrial volumes are being overcome, and that the feasibility of market introduction is rapidly increasing.
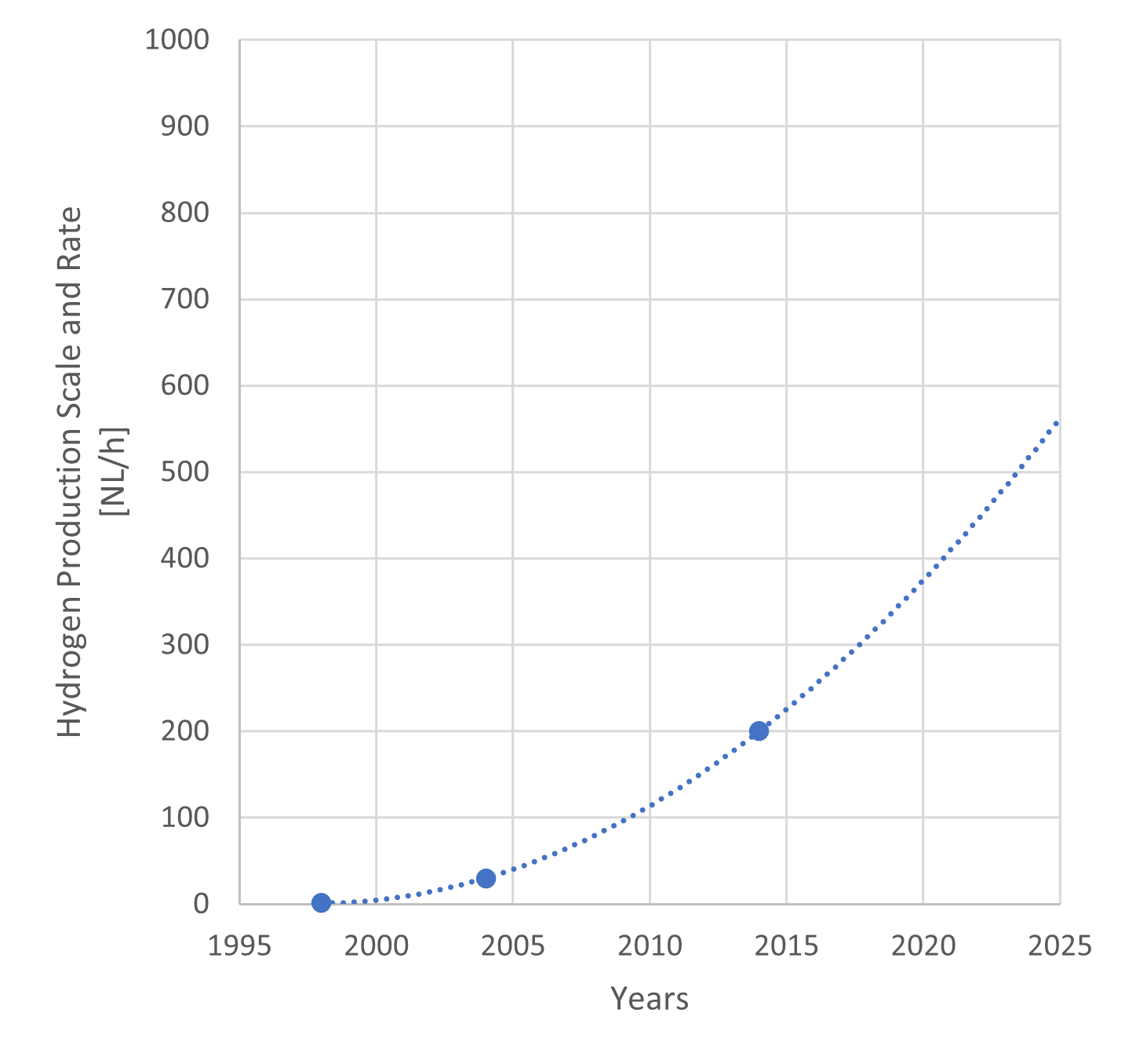
Figure 4.2 illustrates the technological evolution of the High-Temperature Gas-cooled Reactor (HTGR)-Iodine-Sulfur (IS) process. The blue dots represent historical data, showing that the net thermal efficiency has advanced from 32.0% in the early 2000s (actual measurement) to a level exceeding 50% (50.2%) in the latest optimized design calculations (2018). Next, observe the dotted trend line. Based on our logistic growth model, efficiency is projected to reach approximately 55% by around 2040. This projected performance is approximately double the efficiency of conventional water electrolysis (around 30%), shown at the bottom of the graph. This represents an extremely high standard that closely approaches the theoretical thermodynamic limit of 57% shown at the top. In short, this technology holds the promising potential to convert more than half of the input nuclear thermal energy directly into hydrogen energy.
¶
¶ 5. Alignment of Strategic Drivers: FOM Targets
The High-Temperature Gas-cooled Reactor (HTGR) is recognized as an optimal energy source for large-scale, low-cost, carbon-free hydrogen production, owing to its multiple advantages: "high safety," "quasi-domestic energy source (*high energy security through fuel stockpiling)," "high-temperature heat supply capability," and "high energy density."
Furthermore, the IS (Iodine-Sulfur) process, as the production method, holds an overwhelming advantage in terms of domestic resource self-sufficiency. Specifically, whereas blue hydrogen relies on imported raw materials (fossil fuels) and green hydrogen (water electrolysis) depends on imported critical components (precious metals) for its equipment, the IS process can stably procure both its primary raw material, "water," and its circulation media, "iodine" and "sulfur," domestically.
"Our company" will focus on the synergy of these two technologies (HTGR + IS process) and define Japan as our main market. Through this combination, we will establish a clear competitive advantage over other green and blue hydrogen in all four of the following points, and strategically promote a business that contributes to Japan's carbon neutrality and energy security:
1.Production Cost, 2.CO2 Emissions, 3.Annual Availability (Stable Supply), 4.Domestic Resource Ratio (Resource Security)
¶ 6. Positioning of Organization vs. Competition: FOM charts
Hydrogen produced using nuclear power is generally referred to as pink hydrogen. Our company's hydrogen is pink hydrogen, and our competitors are manufacturers of green hydrogen (produced using renewable energy) and blue hydrogen (produced from fossil fuels combined with CCS - Carbon Capture and Storage).
First, regarding blue hydrogen, it is considered difficult to achieve zero CO2 emissions. This gives us a competitive advantage in terms of CO2 emissions. This is because blue hydrogen may incur carbon pricing, such as carbon taxes, due to its CO2 emissions, potentially increasing its production cost. Furthermore, there is a possibility that customers may avoid it.
Second, concerning green hydrogen, especially when produced in Japan, we believe we have an advantage in terms of annual hydrogen production volume. This is due to Japan's limited renewable energy potential. Additionally, green hydrogen production fluctuates throughout the day, making it unsuitable for stable hydrogen supply. Therefore, our pink hydrogen is considered to have a high affinity with manufacturing industries, such as steel and chemicals, which require a stable hydrogen source. Thus, we believe we hold a competitive advantage in the competition for customers within the manufacturing sector.
Additionally, when considering imported green or pink hydrogen from other countries as competitors, it is necessary to account for the production costs in the manufacturing country and the transportation costs. For this reason, it is difficult to make a direct cost comparison at this time.
On the other hand, compared to imported hydrogen, our company has an advantage regarding the domestic self-sufficiency rate. Therefore, it is considered that our company is more aligned with the national strategy.
¶ 7. Technical Model: Morphological Matrix and Tradespace
1. Hydrogen Production rate
Equation
P_th = reactor thermal power, representing the total heat that the reactor can generate.
η_heat = effective heat utilization fraction , expressing how efficiently the reactor heat is delivered to the IS process.
η_rxn = IS reaction efficiency, indicating how effectively the supplied heat is converted into hydrogen through chemical reactions.
HHV (Higher Heating Value) and ρ_H2(Density of Hydrogen) are constants.
The sensitivity analysis showed that all parameters exhibit identical normalized sensitivities (1.0). This indicates that a 1% improvement in any single variable would lead to a 1% increase in hydrogen production, meaning each parameter contributes proportionally to the output.
Nevertheless, while the sensitivities are equal, the cost, time, and technical difficulty required to achieve the same 1% improvement vary significantly. For instance, enhancing heat utilization or reaction efficiency often involves long-term R&D, whereas increasing reactor power can face economic constraints. Considering these practical factors holistically is essential for system design decisions in real world.
2. Hydrogen Production Cost
Equation
where; CH2 : H2 Production Capability, U : Facility Utilization Rate[%], Bavg : Burnup [GWd/t-Uran],
K : Unit Conversion Factor [MJ·kg / GWd·t]
The sensitivity analysis showed that IS reaction efficiency is the most influential factor; thus, JEAE has developed IS-process to improve its efficiency. However, due to thermodynamic bottleneck, its improvement is not easy. On the other hand, Utilization rate, the second most dominant factor, has more relatively rooms for enhancement, including setting proper maintenance interval or introducing advanced process control. While the HTGR-IS process requires huge initial investment, utilization enhancement can decrease the CAPEX impact.
Morphological matrix
Based on the results of the sensitivity analysis, we formulated a Morphological Matrix, which is essential for the comprehensive evaluation of the design space for the integrated HTGR-IS process system. This matrix systematically organizes all feasible combinations by breaking down major design parameters such as the "Core Type," "Intermediate Heat Exchanger (IHX) Design," "Hydrogen Production Plant Layout," and "Key Component Materials
¶ 8. Financial Model: Technology Value (𝛥NPV)
Based on the economic evaluation of HTGR IS process by JAEA (Iwatsuki, J. et al., 2014), financial analysis for the technology focuses on three costs: CAPEX (construction cost), OPEX (maintenance and labor cost) and Fuel cost. The values of each cost, utilization rate and hydrogen production cost refer to the JAEA evaluation, and the price of hydrogen aligns with the Hydrogen Strategy (Japan Ministry of Economy, Trade and Industry, 2023). To simplify the financial model, which includes neglecting revenue and expenses from multiple plants and learning curves, assume: 1. the FOAK is the scope of the analysis, and 2. the plant is under construction for the first 10 years. As the chart below shows, the cash flow will reverse, and the cumulative cash flow will become positive in the 12th year. If the discount rate is 5%, the NPV in the 20th year is ¥ 5,730,842,286.
¶ 9. List of R&D Projects and Prototypes
As the sensitivity analysis in Chapter 7 reveals the impact of each parameter on FOMs, the R&D portfolio should be structured by prioritizing research and development items in the following order: i) hydrogen production efficiency, ii) utilization rate, and iii) thermal output. In addition, safety related R&D projects should be taken into account to the portfolio, as HTGR-IS technology is the first attempt to integrate nuclear power and chemical plants.
i. Hydrogen Production Efficiency
Project Name : Bunsen Reaction Phase-Separation Enhancement
Purpose: Improve phase-separation to raise the purity of HI and H₂SO₄, enhancing overall reaction efficiency.
Development:
- Optimization of anti-emulsification and phase-separation conditions
- Development of additives to promote phase separation
- Fluid behavior optimization (CFD)
ii. Utilization Rate
Project Name : Decomposition Conversion Improvement
Purpose: Increase HI decomposition conversion to maximize hydrogen output.
Development:
- High-temperature durable catalysts (Pt/Ru/Ir)
- Introduction of membrane reactors
- Improved I₂ recovery to shift equilibrium
iii. Thermal Output
Project Name : H₂SO₄ Decomposer Efficiency Improvement
Purpose: Improve high-temperature (850–900°C) H₂SO₄ decomposition efficiency to enhance SO₂ recovery and thermal efficiency.
Development:
- Improvement of high-temperature catalysts
- Uniform temperature distribution design
- Corrosion-resistant materials development
iv. Safety
Project Name : Validation for coupling HTGR with IS plants
Purpose: Validate the safety requirements and PRA methodology developed on paper, and demonstrate the overall system performance.
Development:
- Seismic and Tsunami evaluation
- Connected with HTGR and IS in accordance with established safety standards.
- Hydrogen production with HTGR heat
¶ 10. Key Publications, Presentations and Patents
¶ 10.1 Publications & Presentations
This section compiles key publications and presentations to validate the technological foundation and maturity of the individual component technologies essential for the High-Temperature Gas-cooled Reactor (HTGR)-Iodine-Sulfur (IS) Process integrated system under development. The collected literature supports three critical aspects required for system realization: ① the integrity and performance of the reactor technology (HTTR), ② the early potential for achieving high-efficiency IS process designs, and ③ the demonstration of stable operation for the hydrogen production facility using industrial materials.
"Overview of HTTR design features" (Shiozawa, S. et al., 2004)
This paper summarizes the key design philosophies and features of the High Temperature Engineering Test Reactor (HTTR), designed and constructed by JAEA. The HTTR is a helium-cooled, graphite-moderated reactor with a thermal output of 30 MW and a maximum reactor outlet coolant temperature of 950°C. Since achieving its first criticality in 1998, it has continued to provide data essential for establishing the foundation of HTGR technology through rated-power operations and safety demonstration tests. In the future, there are plans to connect it to a hydrogen production test facility to conduct hydrogen production using nuclear heat.
"HYDROGEN PRODUCTION BY THE GA SULFUR-IODINE PROCESS, A PROGRESS REPORT" (General Atomics, 1980)
This report is a key document summarizing the progress of early research and development by General Atomics (GA). It covers major achievements, including significant improvements in the process's chemical reactions, the development of an engineering flowsheet that achieved 47% thermal efficiency, the screening and testing of materials for corrosive fluids, and the success of a small-scale demonstration operation in a closed-loop cycle. This paper showed that the IS process was a viable technology and provided confidence that thermal efficiencies in the 50% range were achievable.
“Hydrogen production using thermochemical water-splitting Iodine-Sulfur process test facility made of industrial structural materials”(Kubo, S. et al., 2021)
This is one of the important papers summarizing JAEA's (Japan Atomic Energy Agency) recent achievements. It reports the successful continuous operation of a test facility made of industrial structural materials, achieving 30 L/h of hydrogen production over 150 hours.
It is particularly noteworthy for overcoming specific challenges faced in actual plant operation, such as providing an engineering solution to prevent iodine precipitation, which can cause pipe blockages. This demonstrates the integrity of the equipment and the stability of hydrogen production under severe operating conditions.
¶ 10.2 Patents
This section presents a patent search focused on the two main constituent technologies of the HTGR-IS process system under development: the High-Temperature Gas-cooled Reactor (HTGR) unit and the IS hydrogen production process. The objective of this search is to demonstrate that specific technical solutions—such as flowsheet optimization, novel core designs, and improved fuel utilization—necessary for realizing the system economically and with high efficiency, are already protected and publicly disclosed as intellectual property.
US 20240127975 A1 (G21C 1/07, G21C 15/253, G21C 19/202):
This patent describes a serial high-temperature gas-cooled reactor (HTGR) system where spent fuel from multiple primary HTGRs is directly transferred and reused as fuel in a secondary, serial gas-cooled reactor. This "serial" operation is designed to improve the overall utilization rate of nuclear fuel and reduce the system's operating costs.
US 20210375493 A1 (G21C 3/04, G21C 3/28, G21C 15/06):
This patent details a high-temperature gas-cooled reactor (HTGR) core composed of elongate prismatic fuel elements that are uniquely "tall and thin" (height-to-width ratio ≥3.0) with a small cross-sectional area. These elements are arranged in a "multi-lobed prism" configuration , a design which allows for a higher ratio of moderator material within the core to improve reactor efficiency.
US 20130195749 A1 (C01B 3/068):
This patent improves the Iodine-Sulfur (IS) thermochemical cycle for hydrogen production by optimizing the operating conditions of the Bunsen reaction, specifically by minimizing excess water and iodine. This optimization allows the resulting hydrogen iodide (HI) solution to exceed its azeotropic concentration , enabling the use of a simple "flashing process" which lowers energy consumption and reduces equipment corrosion.
WO 2019/140068 A1 (CO1B 3/04, CO1B 17/02, CO1B 17/04):
This invention outlines a process for producing hydrogen gas (H2) and sulfur dioxide (SO2) from hydrogen sulfide (H2 S) and water, using iodine as a recyclable intermediate. The method involves reacting H2 S with iodine and water to create hydroiodic acid (HI) , which is then decomposed to release H2, offering a way to produce valuable hydrogen from a toxic byproduct.
The technology we chose is the system composing HTGR and IS-Process and ; therefore, we could not identify specific one CPC classification in the patent search process, but instead we could identify CPC G21C 1/xx, 3/xx, 15/xx, and 19/xx are related to HTGR system and C01B 3/xx and 17/xx are related to the I-S process.
¶ 11. Technology Strategy Statement
Our company’s R&D objectives are aligned with our mission to begin commercial operation around 2040 and produce competitive, low-cost, stable, and domestically sourced hydrogen. To achieve our strategic goals—hydrogen price of 20 JPY/Nm³ by 2050, zero CO₂ emissions, an operating rate above 90%, and 100% energy self-sufficiency (all raw materials sourced domestically)—we will invest in research and development.
To realize this, we will undertake the following initiatives:
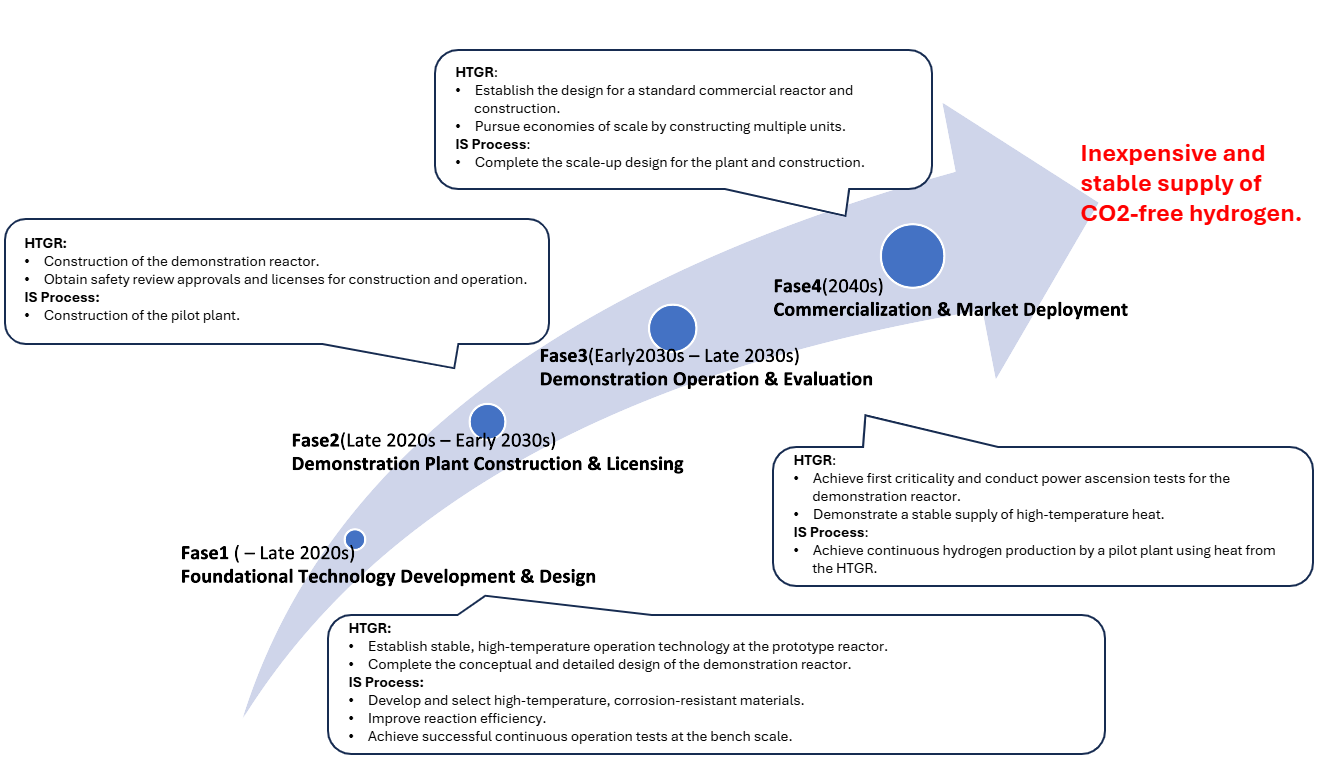
1. Progressive technology maturation through demonstration plants
We will advance technical demonstration in stages—experimental reactor → demonstration reactor → commercial reactor—to ensure the necessary technology readiness level (TRL) for commercial operation in 2040 is achieved.
2. Enhancing the safety and reliability of High-Temperature Gas Reactors (HTGRs)
We will advance core design, material reliability, and passive safety technologies to enable long-term continuous operation.
3. Improving the efficiency of hydrogen production using the IS process
We will maximize hydrogen production efficiency through improved chemical reaction efficiency, development of heat- and corrosion-resistant materials, and overall process optimization.
4. Establishing a domestic supply chain
We will build a system to domestically produce and procure raw materials and components such as helium and Iodine, achieving 100% energy self-sufficiency.
5. Implementing digital technologies to support long-term stable operation
We will utilize AI and digital twin technologies in monitoring and control systems to visualize equipment status and predict failures, maintaining an operating rate above 90%.
6. Supporting regulatory compliance and advancement of safety standards
We will collaborate with regulatory authorities to develop safety standards and contribute to international standardization for the new reactor type and hydrogen production process.
¶ 12. References
- R&D Status on Thermochemical IS Process for Hydrogen Production at JAEA
- JAEA’s R&D on the Thermochemical Hydrogen Production IS Process
- System Evaluation and Economic Analysis of a HTGR Powered High Temperature Electrolysis Hydrogen Production Plant
- Development of High-Temperature Gas-Cooled Reactors for Hydrogen Production by Mitsubishi Heavy Industries
- Introduction of Hydrogen Production Using High-Temperature Gas-Cooled Reactors by JAEA
- IEA, “Global Hydrogen Review”
- Ministry of Economy, Trade and Industry (METI), “Basic Hydrogen Strategy”
- U.S. Department of Energy (DOE), “Hydrogen Shot”
- New Energy and Industrial Technology Development Organization (NEDO), “Fukushima Hydrogen Energy Research Field (FH2R)”
- Air Products, “NEOM Green Hydrogen Complex”
- Stegra, “H2 Green Steel”
- Equinor, “H2H Saltend”
- Constellation Energy, “Constellation and Nel Hydrogen to Build World’s Largest Nuclear-Powered Clean Hydrogen Facility”
- Economic Evaluation of HTGR IS Process Hydrogen Production System (Iwatsuki, J. et al., 2014)
- M. Nomura, S. Kasahara, S. Kubo, et al. (2003). Conceptual design of the pilot plant for hydrogen production by the IS process. Journal of Chemical Engineering of Japan, Vol. 36, No. 7, pp. 887-899.
- Japan Atomic Energy Agency (JAEA). (2014). IS Process Research and Development. JAEA Official Website/Reports.
- S. Kasahara, S. Kubo, T. Tachibana, et al. (2018). Conceptual design of the iodine–sulfur process flowsheet with more than 50% thermal efficiency for hydrogen production. Nuclear Engineering and Design, Vol. 329, pp. 213-222. DOI: 10.1016/j.nucengdes.2017.12.001.