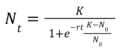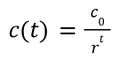Difference between revisions of "Lab Grown Meat"
| (119 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Roadmap Overview== | ==Roadmap Overview== | ||
As technology improves, so does our ability to emulate processes that occur in nature. Human cultivation of food began during the Agricultural Revolution when humans began raising animals in captivity instead of hunting them from the wild. Recently, improvements in cellular agriculture have resulted in the ability to produce meat by directly cultivating the cells instead of cultivating the whole animal. | |||
Taxonomically, cellular agriculture can be categorized as a transform process that applies to organisms, corresponding to (L,1) in the five-by-five Expanded Technology Matrix. This technology takes tissues and cells that ordinarily develop in vivo and provides circumstances that allow them to develop in vitro. | In 2002, researchers funded by NASA cut pieces of muscle from large goldfish, submersed them in fetal bovine serum, and noticed that the cells had multiplied [1]. In 2013, a researcher at Maastricht University announced the production of the first cultured beef patty–which took 2 years and cost over $300,000 to grow [2]. Taste testers determined the patty tasted very close to meat. From 2011 onwards, several startups were founded to commercialize the cultured meat production process, including what is now known as UPSIDE Foods, SuperMeat, GOOD Meats, Believer Meats, and Mosa Meat. The first government-approved cultivated meat was produced by Eat Just, approved by Singapore to sell cultured chicken, in 2020. | ||
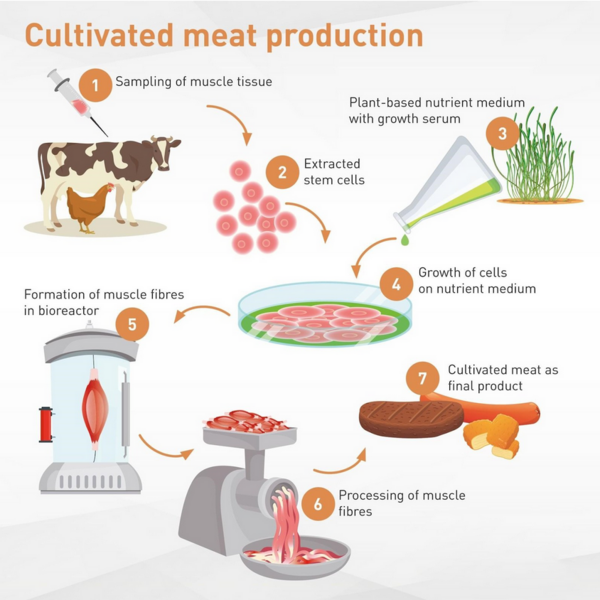
Presently, the industry at large faces the challenge of shifting from an early research and development phase to scaling up the production processes for their cultivated meat. This roadmap focuses on cellular agriculture for meat production, which is also commonly referred to as cultured meat or lab-grown meat. This technology permits one to generate edible meat products without rearing and slaughtering livestock. Instead, operators can obtain targeted tissue and cell samples from the desired organism and then culture them in a bioreactor to fabricate meat products that are biologically identical and structurally similar to their conventional counterparts. Anticipated benefits of this technology are increased water efficiency, reduced feed requirements, and lower risks of zoonotic disease transfer–and, as a consequence, reduced emissions and harm to the environment, and avoidance of harm to live animals. | |||
The abbreviated identifier for this technology roadmap is 2LGM. The 2 describes a level 2, product-level roadmap. LGM stands for Lab Grown Meat. Taxonomically, cellular agriculture can be categorized as a transform process that applies to organisms, corresponding to (L,1) in the five-by-five Expanded Technology Matrix. This technology takes tissues and cells that ordinarily develop in vivo and provides circumstances that allow them to develop in vitro. | |||
*'''Tissue Engineering Applications (1TEA)''' | *'''Tissue Engineering Applications (1TEA)''' | ||
| Line 26: | Line 30: | ||
**4TPL: Transparent Labeling | **4TPL: Transparent Labeling | ||
**4RDC: Religious Diet Certification | **4RDC: Religious Diet Certification | ||
[[File:LGM_cartoon.png|600px]] | |||
'''Sources:''' | |||
[1] https://www.newscientist.com/article/dn2066-fish-fillets-grow-in-tank/ | |||
[2] https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html | |||
[3] https://www.four-paws.org/campaigns-topics/topics/nutrition/cultivated-meat-food-innovation/what-is-cultivated-meat | |||
==Design Structure Matrix (DSM) Allocation== | ==Design Structure Matrix (DSM) Allocation== | ||
[[File: | A hierarchy of related technologies is provided below and explains their relationship with our lab grown meat roadmap (2LGM). 2LGM is classified as a type of tissue engineering technology, or 1TEA, which is a level 1 market-level roadmap. Other types of tissue engineering technology include organ regeneration (2ORG) and artificial wombs (2ARW). Under 2LGM are level 3 subcomponent roadmaps, including cell-tissue culturing (3CTC) and cell-line development (3CEL). Under these roadmaps are level 4 individual component roadmap, including the bioreactor (4BIR). | ||
[[File:LGM_DSM2.png|720px]] | |||
==Roadmap Model using OPM== | ==Roadmap Model using OPM== | ||
Techno-System OPD for Lab Grown Meat | '''Techno-System OPD for Lab Grown Meat''' | ||
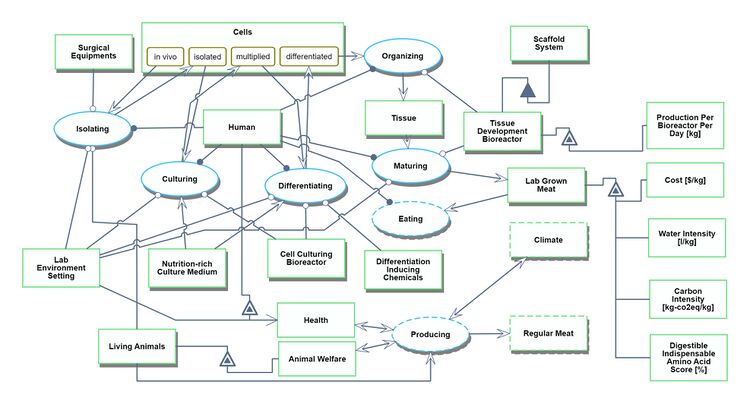
We provide two Object Process Diagrams (OPD) below for our Lab Grown Meat (2LGM) roadmap. The first is the techno-system OPD, which explains the process itself of producing lab-grown meat–including the creation of the bioreactor, the donor living animals, the procurement of the culture medium, and the surgical equipment. The Figures of Merit (FOMs) are displayed on the right side. | |||
[[File: | [[File:LGM_OPD_technosys.jpg|750px]] | ||
[[File:LGM_OPL_technosys.png|750px]] | |||
----------------------------------- | |||
'''Roadmapping OPD for Lab Grown Meat''' | |||
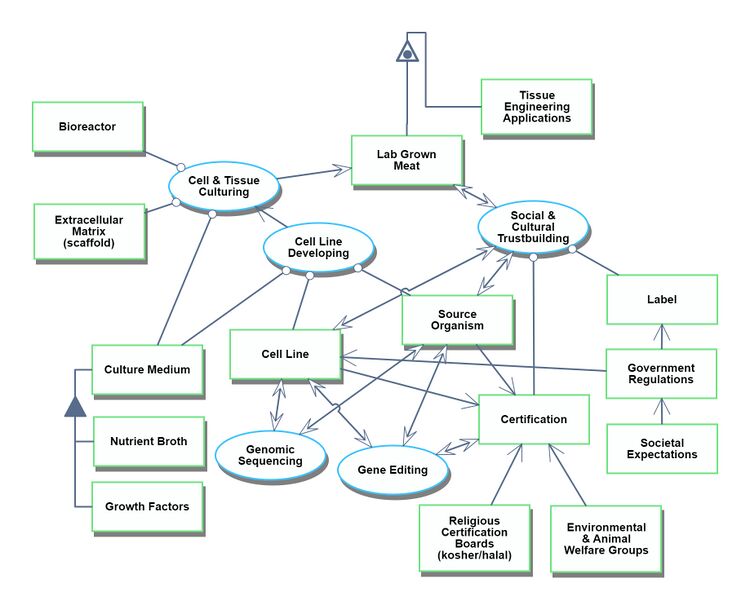
[[File:LGM_OPL_roadmap.png| | Our second OPD focuses on the development process for the technological capability to produce lab grown meat. This includes the relationship of lab grown meat technology to social and cultural institutions. For example, the finished lab grown meat must be certified by religious or environmental boards, and must be approved by government regulations. | ||
[[File:LGM_OPD_roadmap.jpg|750px]] | |||
[[File:LGM_OPL_roadmap.png|750px]] | |||
==Figures of Merit== | ==Figures of Merit== | ||
[[File: | The Figures of Merit (FOMs) of Lab Grown Meat (2LGM) are provided below. These are also displayed in the first Object Process Diagram (OPD) above. | ||
[[File:LGM_FOM_2.png|650px]] | |||
It should be noted that the majority of these FOMs are important for the production process and should be considered by every company when scaling up production. However, few existing companies have publicly reported FOMs–some information is available on the cost of production and the production per bioreactor per day, but the carbon or water intensity of production is not reported. As a result, the remainder of our report focuses on the cost of production. | |||
'''Sources:''' | |||
[1] https://ourworldindata.org/environmental-impacts-of-food | |||
[2] https://www.theguardian.com/news/datablog/2013/jan/10/how-much-water-food-production-waste | |||
[3] https://www.ers.usda.gov/data-products/commodity-costs-and-returns/commodity-costs-and-returns/#Historical%20Costs%20and%20Returns:%20Cow-Calf | |||
[4] https://beef.unl.edu/beefwatch/2020/how-many-pounds-meat-can-we-expect-beef-animal | |||
[5] https://www.sciencedirect.com/science/article/pii/S1751731116000902?via%3Dihub | |||
==Alignment with Strategic Drivers== | ==Alignment with Strategic Drivers== | ||
Current companies producing lab grown meat are | In this section we provide a market summary. According to a McKinsey report from 2021 [1], the industry consisted of under 100 startups which had attracted over $350 in investments in 2020 and $250 million in 2021. The market for cultivated meat could reach as high as $25 billion by 2030. | ||
[[File:LGM_market_projections.png|650px]] | |||
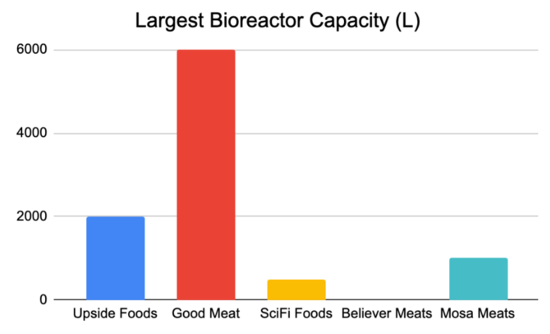
Current companies producing lab grown meat are UPSIDE Foods, Believer Meats, and GOOD Meat. GOOD Meat has the single largest bioreactor for cultivated meat at 6000 L in size. After comparing and contrasting the strategic drivers of these other companies, we can create our own: | |||
[[File:alignment2.png|700px]] | |||
For reference, the strategic drivers of GOOD Meat include driving “their serum-free media cost further down from the current $1 per liter to ‘tens of cents,’ according to Tetrick, allowing the company to produce “hundreds of thousands of pounds” of cultivated meat.” GOOD Meat’s “next phase is to install vessels north of 100,000 liter each, which will enable tens of millions of pounds.” GOOD Meat is also the first company to start selling chicken nuggets and chicken breasts through restaurants and food vendors in Singapore, and dishes cost about $18.5 singaporean dollars (or $14 USD). [2] | |||
[ | Believer Meats, another startup from Israel, “recently claimed to have achieved a production density of 100 billion cells per liter,” in addition to using a “medium that costs less than $5 per liter.” [2] | ||
'''Sources:''' | |||
[1] https://www.mckinsey.com/industries/agriculture/our-insights/cultivated-meat-out-of-the-lab-into-the-frying-pan | |||
[ | [2] https://www.forbes.com/sites/douglasyu/2023/01/18/eat-just-to-scale-up-cultured-meat-production-on-gaining-new-regulatory-approval-in-singapore/?sh=70b8476349d7 | ||
==Position of | ==Position of Companies== | ||
[[File:FGM_position6.png|660px]] | |||
[[File:FGM_bioreactor_cap.png|550px]] | |||
[[File:FGM_prod_cost.png|550px]] | |||
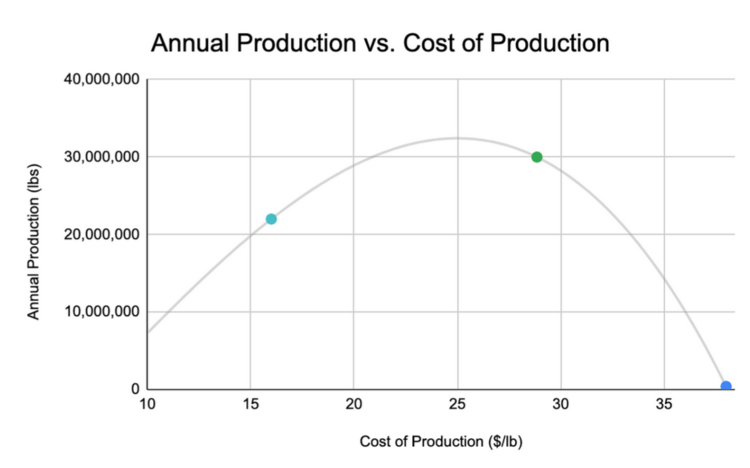
[[File:FGM_annual_production.png|550px]] | |||
'''NOTE:''' As this is an emerging market, details concerning company metrics and plans are sparse and fairly vague. | |||
'''Sources:''' | |||
[1] https://cen.acs.org/food/Inside-effort-cut-cost-cultivated/101/i33 | |||
[2] https://www.trouw.nl/nieuws/van-het-lab-naar-een-bord-is-een-lange-weg-voor-kweekvlees~be6d9867/ | |||
[3] https://www.fooddive.com/news/upside-foods-cultivated-cell-based-meat-plant-epic/609182/ | |||
[4] https://www.illinois.gov/news/press-release.27020.html | |||
[5] https://techcrunch.com/2023/01/18/good-meat-regulatory-approval/ | |||
[6] https://agfundernews.com/good-meat-gets-green-light-from-fda-for-cultivated-meat | |||
[7] https://www.foodnavigator-usa.com/Article/2022/05/25/GOOD-Meat-plans-US-cultivated-meat-facility-with-capacity-to-produce-up-to-30m-lbs-of-meat# | |||
[8] https://www.fastcompany.com/90831145/the-first-crispr-gene-edited-meat-is-coming-and-this-is-the-ceo-making-sci-fi-reality | |||
[9] https://www.mckinsey.com/industries/agriculture/our-insights/cultivated-meat-out-of-the-lab-into-the-frying-pan | |||
[10] https://www.prnewswire.com/news-releases/believer-meats-breaks-ground-on-largest-cultivated-meat-production-facility-in-the-world-301697617.html | |||
[11] https://agfundernews.com/mosa-meat-opens-facility-sees-a-clear-path-towards-price-parity | |||
[12] https://cleantechnica.com/2019/09/12/mosa-meat-from-e250000-to-e9-burger-patties/ | |||
[13] https://www.dutchnews.nl/2020/10/food-for-thought-cultured-meat-maker-brings-in-55m-in-funding/ | |||
==Technical Model== | ==Technical Model== | ||
'''Governing Equation''' | |||
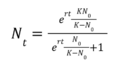
The process of lab grown meat production is very complicated and there are no obvious governing equations that precisely describe the technical process. However, we can abstract a basic governing equation for the cell growth rate to calculate the rate of change of the growth rate of a population of cells as the basis for cell replication in the culture medium. The dN/dt, or rate of change of population size, then becomes our first FOM. | |||
[[File:LGM_gov_eq.png|120px]] | |||
Where: | |||
- N is the population size at time, t | |||
- dN/dt represents the rate of change of population size, | |||
- r is the theoretical growth rate of the cells in a hypothetical ideal environment with unlimited resources | |||
- K is the carrying capacity of the environment (the maximum possible population size in the environment) | |||
After formula transformation, it can be expressed in integral form: | |||
[[File:LGM_other_gov_eq.png|120px]] | |||
'''Tradespace''' | '''Tradespace''' | ||
[[File:LGM_tradespace2.png|750px]] | |||
The market tradespace for current lab-grown meat companies. The blue datapoint is Believer Meats, the red datapoint is Upside Meats, and the green datapoint is Mosa Meats. Note that the two axes, the planned cost, and planned production, are taken from news releases from all three startups. The further left a data point is, the lower the planned cost, and the more desirable the technology. Similarly, the further up a data point is, the higher the planned production, and the more desirable the technology. | |||
To optimize the technology of lab-grown meat, we seek technologies towards the top left of this tradespace. | To optimize the technology of lab-grown meat, we seek technologies towards the top left of this tradespace. | ||
| Line 78: | Line 180: | ||
Unfortunately, there is a lack of data due to the nature of the industry as one of developing technologies which have not begun to release data and for which production methods are like trade secrets. Since we have so few data points, it is also difficult to assess the pareto frontier of what is achievable in production and cost. | Unfortunately, there is a lack of data due to the nature of the industry as one of developing technologies which have not begun to release data and for which production methods are like trade secrets. Since we have so few data points, it is also difficult to assess the pareto frontier of what is achievable in production and cost. | ||
One potential reason that Believer Meats (formerly known as Future Meat) has such a high production and low cost per pound is because of their business model, where they mix lab grown meat with plant-based proteins, which are a lot cheaper to produce. | One potential reason that Believer Meats (formerly known as Future Meat Technologies) has such a high production and low cost per pound is because of their business model, where they mix lab grown meat with plant-based proteins, which are a lot cheaper to produce. | ||
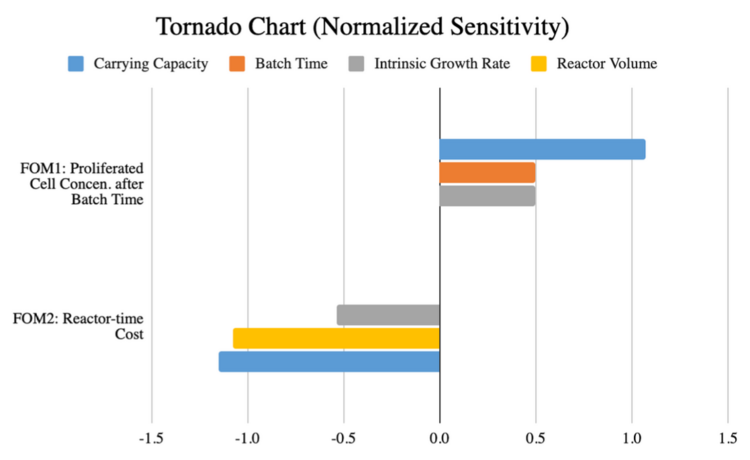
'''Tornado Chart for Sensitivity Analysis of Selected FOMs''' | |||
[[File:LGM_tornado_combined.png|750px]] | |||
First FOM, Nt (concentration of cells proliferated after a batch time t, [cells/mL]): | |||
[[File:LGM_1stfom.png|120px]] | |||
With the influential parameters being K (carrying capacity [cells/mL]), t (batch time [day]), r (intrinsic growth rate [day-1]). | |||
Second FOM, J (reactor-day cost of producing 1 lb of beef, [reactor-days]): | |||
[[File:LGM_2ndfom.png|200px]] | |||
Where n = 10^14 cells per pound of beef | |||
And the influential parameters being V (reactor volume [mL]), K (carrying capacity [cells/mL]), r (intrinsic growth rate [day-1]). | |||
==Key Publications and Patents == | ==Key Publications and Patents == | ||
[[File: | '''Key Patents''' | ||
[[File:patents2.png|650px]] | |||
'''Key Papers''' | |||
[[File:papers2.png|650px]] | |||
==Financial Model== | |||
The financial model is based on NPV analysis of nonrecurring cost, revenues and manufacturing recurring cost. And we select UPSIDE Foods (formerly known as Memphis Meats) for case study here. UPSIDE Foods is the first (and one of the only two for now) startup company dedicated to lab-grown meat production that has got approval from FDA. As it is a startup that pioneers the innovation of a new technology, the impacts relative to a baseline business plan can be quantified with the absolute values of costs and revenues. | |||
In accordance with the example provided in Chapter 8 of the textbook, the impacts can be broken down into an initial period (with no scaled production), a ramp-up period and a plateau period after reaching market equilibrium. | |||
For the intuitiveness of analysis, we hypothetically assume that we are currently in the year before UPSIDE Foods was set up. | |||
[[File:LGM_fin_model.jpg|800px]] | |||
The components of such a financial model are: | |||
1. Initial period: nonrecurring cost | |||
2. Ramp-up period: manufacturing cost and revenue, variable | |||
3. Plateau period: manufacturing recurring cost and revenue, constant | |||
According to news report, the initial cost for R&D and infrastructure of scaled up production was about $140M for UPSIDE Foods [1], and the company was established in 2014 while started making revenues from production in 2017. So we scaled the initial cost to a 3-year initial period and got the nonrecurring cost value of $47M every year. | |||
The projected revenue in the plateau period can be calculated with the planned capacity and unit price of the product. UPSIDE Foods plans to scale up to a total capacity of 400,000 lbs [2]. The unit price of lab-grown meat should be comparable to the traditional meat products in the plateau period, which means we can assume it to be the highest price in the current meat market, $12/lb [3]. This leads to an annual revenue of $4.8M [4] during the plateau period. | |||
We assume a Logistic Growth Model for the revenues in the ramp-up period. The expression of such a model is: | |||
[[File:LGM_equation1.jpg|120px]] | |||
where c is the maximum revenue ($4.8M). The other parameters a and b can be derived by looking at two reference values, $0.001M for 2017 and $2.8M for 2022 [4]. This gives us a=4799 and b=1.7627. By setting f(t) =$4.8M-$0.0001M, we can solve for the length of ramp-up period t=10, meaning the model enters the plateau period in 2027. | |||
For manufacturing cost, we assume an exponential decay of unit production cost: | |||
[[File:LGM_equation2.jpg|120px]] | |||
Using reference values $18,000/lb (2014) [5], $9,000/lb (2016) [6], $2,400/lb (2017) [7], we can calculate the cost decay factor r=1.96. And according to food technology researchers, the unit production cost has to go down to $3 for lab-grown meat to be competitive in the real meat product market [8]. Inserting this value into the exponential decay function we fitted, we find that the plateau period should start in 2027,which agrees with the derivation for revenues. The production capacity as of 2022 was 50,000 lbs, and if we assume linear growth from 2017 to 2022, and from 2022 to 2027, we can impute the capacity for each year and calculate the annual manufacturing recurring cost by multiplying capacity and unit production cost. | |||
'''Sources:''' | |||
[1] https://agfundernews.com/upside-foods-to-pump-140m-into-commercial-scale-cultivated-meat-plant-near-chicago | |||
[2] https://upsidefoods.com/innovation | |||
[3] https://www.ers.usda.gov/data-products/meat-price-spreads/ | |||
[4] https://www.zippia.com/memphis-meats-careers-1392392/revenue/ | |||
[5] https://www.wsj.com/articles/startup-to-serve-up-chicken-strips-cultivated-from-cells-in-lab-1489570202 | |||
[6] https://www.foxnews.com/leisure/2016/02/03/world-first-lab-grown-meatball-revealed/ | |||
[7] https://www.wsj.com/articles/cargill-backs-cell-culture-meat-1503486002 | |||
[8] https://www.reuters.com/markets/commodities/cell-cultivated-meat-hits-menus-investors-see-scaling-next-hurdle-2023-07-20/ | |||
==R&D Projects== | |||
1. Scaling up operations for mass market (TRL6) - Over 100 start-ups are currently working to mass produce and commercialize affordable lab-grown meat products, which currently exist only as a small-scale and high-cost products available in limited retail contexts. | |||
2. Growth factor-free cell lines (TRL4) - Many companies seek to genetically engineer cell lines to produce their own growth factors, eliminating the need for expensive, additive growth factors. | |||
3. Added scaffold-free cell lines (TRL2) - Similar to growth factors, several companies are beginning to work on genetically engineer cell lines to produce their own scaffold, which reduces the need for added, external scaffolds. | |||
----- | |||
'''Demonstrator Projects''' | |||
'''University of Maastricht burger (2013):''' | |||
[[File:first_LGM_burger.jpg|500px]] [1] | |||
Dr. Mark Post unveiled a five-ounce cultured meat patty created with mulitpotent stem cells (myosatellite cells) as a public-facing proof-of-concept. Post's patty required nearly 20,000 strips of tissue to construct. The $300,000-plus patty received extensive press coverage and tolerant taste reviews. [1,2] | |||
[[File:LGM_headline.png|500px]] [2] | |||
'''Vow’s Mammoth Meatball (2023):''' | |||
[[File:mammoth_meatball.jpg|500px]] [3] | |||
[[File:mammoth_webpage.png|500px]] [4] | |||
Using publicly-available DNA data, Vow isolated the gene that coded for myoglobin in woolly mammoths and filled gaps with African elephant DNA. This reconstructed mammoth myoglobin gene was then inserted into muscle cells taken from sheep. Because sheep and mammoths are more distantly related, technicians could easily identify which sheep cells were now producing mammoth myoglobin. The cells expressing mammoth myoglobin were proliferated (to approximately 20 billion cells) before processing into the mammoth meatball which was exhibited at a Dutch science museum. [3] | |||
'''Sources:''' | |||
[1] https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html | |||
[2] https://www.washingtonpost.com/national/health-science/lab-grown-beef-taste-test-almost-like-a-burger/2013/08/05/921a5996-fdf4-11e2-96a8-d3b921c0924a_story.html | |||
[3] https://www.theguardian.com/environment/2023/mar/28/meatball-mammoth-created-cultivated-meat-firm | |||
[4] https://www.mammothmeatball.com/the-recipe | |||
==Technology Statement and Swoosh Chart== | |||
Since lab-grown meat is a new industry with a low TRL of around 4-6, our technology strategy in the near-term future focuses on improving the technology for the production of lab grown meat and scaling it to a level where production can become profitable, and startups can begin selling their products. | |||
Our main strategic drivers are to (1) reduce growth factor costs and the amount of growth medium needed, (2) scale up individual bioreactor capacity to improve output, (3) achieve efficient bioreactor design and techniques for a sustainable production rate. (4) market lab-grown meat at a price competitive with conventional meat. | |||
[[File: | [[File:LGM_swoosh_new2.png|920px]] | ||
Latest revision as of 15:47, 5 December 2023
Roadmap Overview
As technology improves, so does our ability to emulate processes that occur in nature. Human cultivation of food began during the Agricultural Revolution when humans began raising animals in captivity instead of hunting them from the wild. Recently, improvements in cellular agriculture have resulted in the ability to produce meat by directly cultivating the cells instead of cultivating the whole animal.
In 2002, researchers funded by NASA cut pieces of muscle from large goldfish, submersed them in fetal bovine serum, and noticed that the cells had multiplied [1]. In 2013, a researcher at Maastricht University announced the production of the first cultured beef patty–which took 2 years and cost over $300,000 to grow [2]. Taste testers determined the patty tasted very close to meat. From 2011 onwards, several startups were founded to commercialize the cultured meat production process, including what is now known as UPSIDE Foods, SuperMeat, GOOD Meats, Believer Meats, and Mosa Meat. The first government-approved cultivated meat was produced by Eat Just, approved by Singapore to sell cultured chicken, in 2020.
Presently, the industry at large faces the challenge of shifting from an early research and development phase to scaling up the production processes for their cultivated meat. This roadmap focuses on cellular agriculture for meat production, which is also commonly referred to as cultured meat or lab-grown meat. This technology permits one to generate edible meat products without rearing and slaughtering livestock. Instead, operators can obtain targeted tissue and cell samples from the desired organism and then culture them in a bioreactor to fabricate meat products that are biologically identical and structurally similar to their conventional counterparts. Anticipated benefits of this technology are increased water efficiency, reduced feed requirements, and lower risks of zoonotic disease transfer–and, as a consequence, reduced emissions and harm to the environment, and avoidance of harm to live animals.
The abbreviated identifier for this technology roadmap is 2LGM. The 2 describes a level 2, product-level roadmap. LGM stands for Lab Grown Meat. Taxonomically, cellular agriculture can be categorized as a transform process that applies to organisms, corresponding to (L,1) in the five-by-five Expanded Technology Matrix. This technology takes tissues and cells that ordinarily develop in vivo and provides circumstances that allow them to develop in vitro.
- Tissue Engineering Applications (1TEA)
- 2ORG: Organ Regeneration
- 2VOF: In Vitro Organ Fabrication
- 2IVM: In Vitro Model
- 2OOC: Organ-on-a-Chip
- 2ARW: Artificial Womb
- 2LGM: Lab-Grown Meat
- Lab-Grown Meat (2LGM)
- 3CTC: Cell/Tissue Culturing
- 3CEL: Cell Line Development
- 3SCA: Socio-Cultural Acceptance
- Cell/Tissue Culturing (3CTC)
- 4BIR: Bioreactor
- 4MED: Culture Medium
- 4ECM: Extracellular Matrix (Scaffold)
- Cell Line Development (3CEL)
- 4SOR: Source Organism
- 4CPD: Cell Proliferation and Differentiation
- Socio-Cultural Acceptance (3SCA)
- 4TPL: Transparent Labeling
- 4RDC: Religious Diet Certification
Sources:
[1] https://www.newscientist.com/article/dn2066-fish-fillets-grow-in-tank/
[2] https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html
Design Structure Matrix (DSM) Allocation
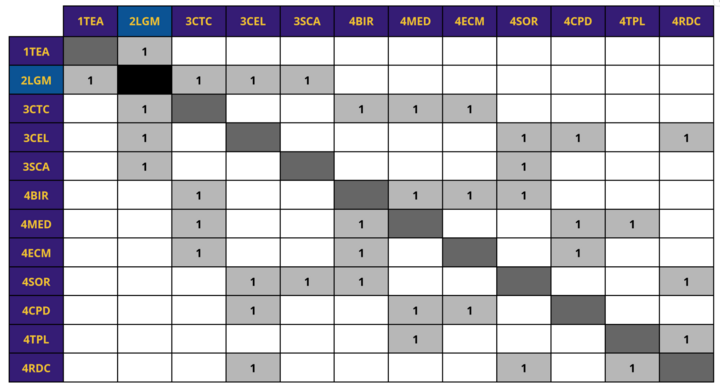
A hierarchy of related technologies is provided below and explains their relationship with our lab grown meat roadmap (2LGM). 2LGM is classified as a type of tissue engineering technology, or 1TEA, which is a level 1 market-level roadmap. Other types of tissue engineering technology include organ regeneration (2ORG) and artificial wombs (2ARW). Under 2LGM are level 3 subcomponent roadmaps, including cell-tissue culturing (3CTC) and cell-line development (3CEL). Under these roadmaps are level 4 individual component roadmap, including the bioreactor (4BIR).
Roadmap Model using OPM
Techno-System OPD for Lab Grown Meat
We provide two Object Process Diagrams (OPD) below for our Lab Grown Meat (2LGM) roadmap. The first is the techno-system OPD, which explains the process itself of producing lab-grown meat–including the creation of the bioreactor, the donor living animals, the procurement of the culture medium, and the surgical equipment. The Figures of Merit (FOMs) are displayed on the right side.
Roadmapping OPD for Lab Grown Meat
Our second OPD focuses on the development process for the technological capability to produce lab grown meat. This includes the relationship of lab grown meat technology to social and cultural institutions. For example, the finished lab grown meat must be certified by religious or environmental boards, and must be approved by government regulations.
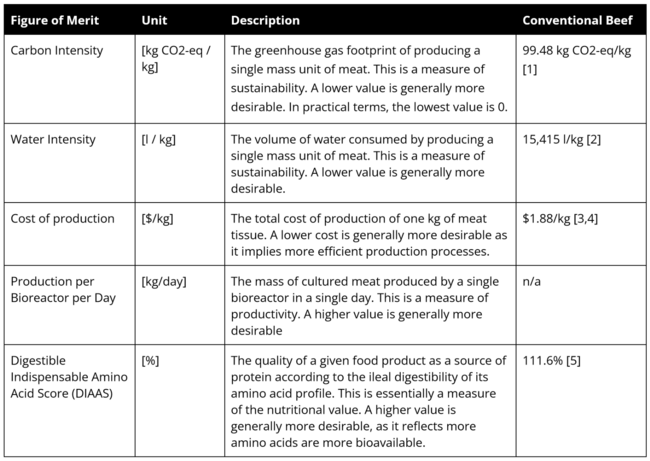
Figures of Merit
The Figures of Merit (FOMs) of Lab Grown Meat (2LGM) are provided below. These are also displayed in the first Object Process Diagram (OPD) above.
It should be noted that the majority of these FOMs are important for the production process and should be considered by every company when scaling up production. However, few existing companies have publicly reported FOMs–some information is available on the cost of production and the production per bioreactor per day, but the carbon or water intensity of production is not reported. As a result, the remainder of our report focuses on the cost of production.
Sources:
[1] https://ourworldindata.org/environmental-impacts-of-food
[2] https://www.theguardian.com/news/datablog/2013/jan/10/how-much-water-food-production-waste
[4] https://beef.unl.edu/beefwatch/2020/how-many-pounds-meat-can-we-expect-beef-animal
[5] https://www.sciencedirect.com/science/article/pii/S1751731116000902?via%3Dihub
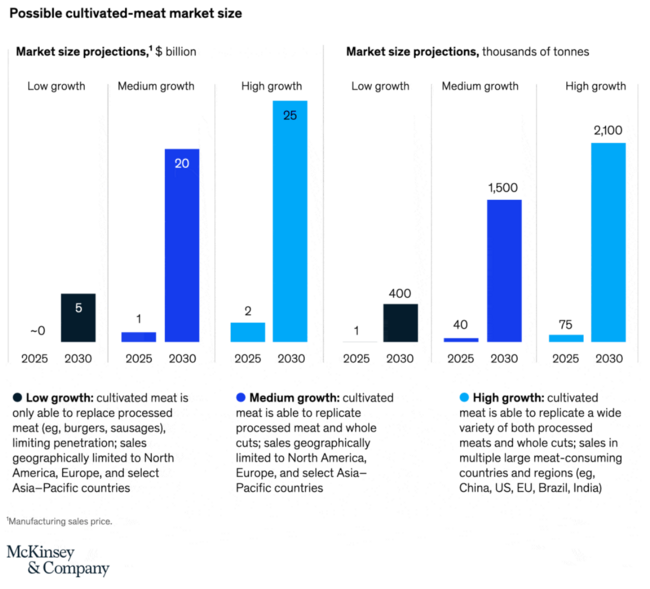
Alignment with Strategic Drivers
In this section we provide a market summary. According to a McKinsey report from 2021 [1], the industry consisted of under 100 startups which had attracted over $350 in investments in 2020 and $250 million in 2021. The market for cultivated meat could reach as high as $25 billion by 2030.
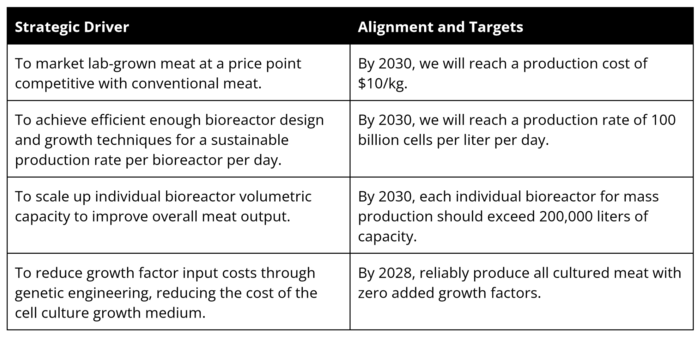
Current companies producing lab grown meat are UPSIDE Foods, Believer Meats, and GOOD Meat. GOOD Meat has the single largest bioreactor for cultivated meat at 6000 L in size. After comparing and contrasting the strategic drivers of these other companies, we can create our own:
For reference, the strategic drivers of GOOD Meat include driving “their serum-free media cost further down from the current $1 per liter to ‘tens of cents,’ according to Tetrick, allowing the company to produce “hundreds of thousands of pounds” of cultivated meat.” GOOD Meat’s “next phase is to install vessels north of 100,000 liter each, which will enable tens of millions of pounds.” GOOD Meat is also the first company to start selling chicken nuggets and chicken breasts through restaurants and food vendors in Singapore, and dishes cost about $18.5 singaporean dollars (or $14 USD). [2]
Believer Meats, another startup from Israel, “recently claimed to have achieved a production density of 100 billion cells per liter,” in addition to using a “medium that costs less than $5 per liter.” [2]
Sources:
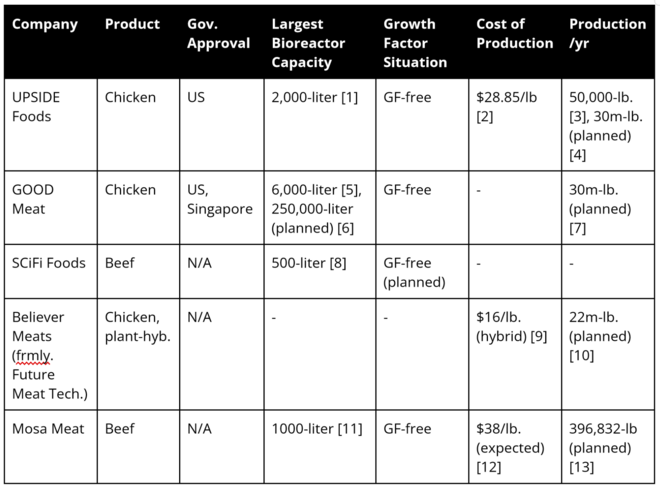
Position of Companies
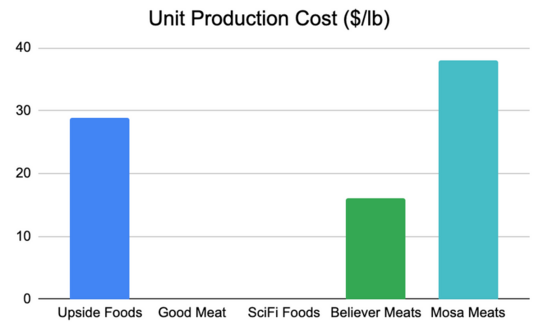
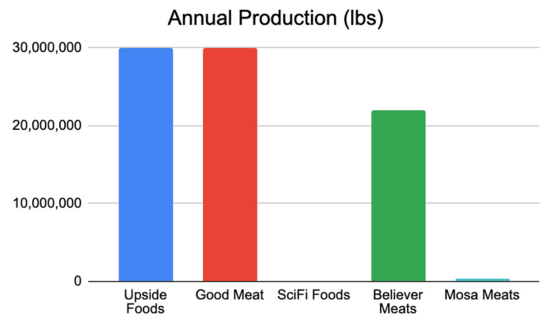
NOTE: As this is an emerging market, details concerning company metrics and plans are sparse and fairly vague.
Sources:
[1] https://cen.acs.org/food/Inside-effort-cut-cost-cultivated/101/i33
[2] https://www.trouw.nl/nieuws/van-het-lab-naar-een-bord-is-een-lange-weg-voor-kweekvlees~be6d9867/
[3] https://www.fooddive.com/news/upside-foods-cultivated-cell-based-meat-plant-epic/609182/
[4] https://www.illinois.gov/news/press-release.27020.html
[5] https://techcrunch.com/2023/01/18/good-meat-regulatory-approval/
[6] https://agfundernews.com/good-meat-gets-green-light-from-fda-for-cultivated-meat
[11] https://agfundernews.com/mosa-meat-opens-facility-sees-a-clear-path-towards-price-parity
[12] https://cleantechnica.com/2019/09/12/mosa-meat-from-e250000-to-e9-burger-patties/
[13] https://www.dutchnews.nl/2020/10/food-for-thought-cultured-meat-maker-brings-in-55m-in-funding/
Technical Model
Governing Equation
The process of lab grown meat production is very complicated and there are no obvious governing equations that precisely describe the technical process. However, we can abstract a basic governing equation for the cell growth rate to calculate the rate of change of the growth rate of a population of cells as the basis for cell replication in the culture medium. The dN/dt, or rate of change of population size, then becomes our first FOM.
Where:
- N is the population size at time, t
- dN/dt represents the rate of change of population size,
- r is the theoretical growth rate of the cells in a hypothetical ideal environment with unlimited resources
- K is the carrying capacity of the environment (the maximum possible population size in the environment)
After formula transformation, it can be expressed in integral form:
Tradespace
The market tradespace for current lab-grown meat companies. The blue datapoint is Believer Meats, the red datapoint is Upside Meats, and the green datapoint is Mosa Meats. Note that the two axes, the planned cost, and planned production, are taken from news releases from all three startups. The further left a data point is, the lower the planned cost, and the more desirable the technology. Similarly, the further up a data point is, the higher the planned production, and the more desirable the technology.
To optimize the technology of lab-grown meat, we seek technologies towards the top left of this tradespace.
Unfortunately, there is a lack of data due to the nature of the industry as one of developing technologies which have not begun to release data and for which production methods are like trade secrets. Since we have so few data points, it is also difficult to assess the pareto frontier of what is achievable in production and cost.
One potential reason that Believer Meats (formerly known as Future Meat Technologies) has such a high production and low cost per pound is because of their business model, where they mix lab grown meat with plant-based proteins, which are a lot cheaper to produce.
Tornado Chart for Sensitivity Analysis of Selected FOMs
First FOM, Nt (concentration of cells proliferated after a batch time t, [cells/mL]):
With the influential parameters being K (carrying capacity [cells/mL]), t (batch time [day]), r (intrinsic growth rate [day-1]).
Second FOM, J (reactor-day cost of producing 1 lb of beef, [reactor-days]):
Where n = 10^14 cells per pound of beef
And the influential parameters being V (reactor volume [mL]), K (carrying capacity [cells/mL]), r (intrinsic growth rate [day-1]).
Key Publications and Patents
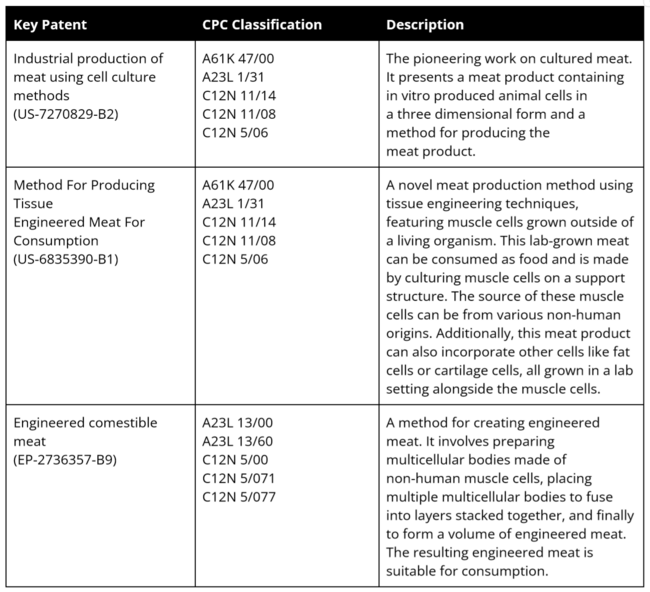
Key Patents
Key Papers
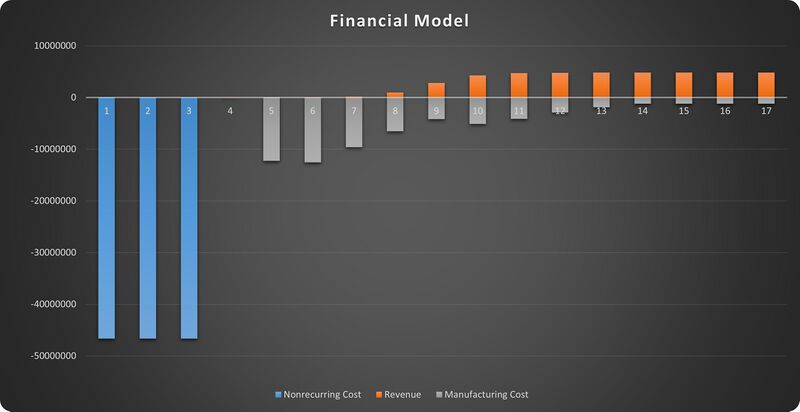
Financial Model
The financial model is based on NPV analysis of nonrecurring cost, revenues and manufacturing recurring cost. And we select UPSIDE Foods (formerly known as Memphis Meats) for case study here. UPSIDE Foods is the first (and one of the only two for now) startup company dedicated to lab-grown meat production that has got approval from FDA. As it is a startup that pioneers the innovation of a new technology, the impacts relative to a baseline business plan can be quantified with the absolute values of costs and revenues.
In accordance with the example provided in Chapter 8 of the textbook, the impacts can be broken down into an initial period (with no scaled production), a ramp-up period and a plateau period after reaching market equilibrium.
For the intuitiveness of analysis, we hypothetically assume that we are currently in the year before UPSIDE Foods was set up.
The components of such a financial model are:
1. Initial period: nonrecurring cost
2. Ramp-up period: manufacturing cost and revenue, variable
3. Plateau period: manufacturing recurring cost and revenue, constant
According to news report, the initial cost for R&D and infrastructure of scaled up production was about $140M for UPSIDE Foods [1], and the company was established in 2014 while started making revenues from production in 2017. So we scaled the initial cost to a 3-year initial period and got the nonrecurring cost value of $47M every year.
The projected revenue in the plateau period can be calculated with the planned capacity and unit price of the product. UPSIDE Foods plans to scale up to a total capacity of 400,000 lbs [2]. The unit price of lab-grown meat should be comparable to the traditional meat products in the plateau period, which means we can assume it to be the highest price in the current meat market, $12/lb [3]. This leads to an annual revenue of $4.8M [4] during the plateau period.
We assume a Logistic Growth Model for the revenues in the ramp-up period. The expression of such a model is:
where c is the maximum revenue ($4.8M). The other parameters a and b can be derived by looking at two reference values, $0.001M for 2017 and $2.8M for 2022 [4]. This gives us a=4799 and b=1.7627. By setting f(t) =$4.8M-$0.0001M, we can solve for the length of ramp-up period t=10, meaning the model enters the plateau period in 2027.
For manufacturing cost, we assume an exponential decay of unit production cost:
Using reference values $18,000/lb (2014) [5], $9,000/lb (2016) [6], $2,400/lb (2017) [7], we can calculate the cost decay factor r=1.96. And according to food technology researchers, the unit production cost has to go down to $3 for lab-grown meat to be competitive in the real meat product market [8]. Inserting this value into the exponential decay function we fitted, we find that the plateau period should start in 2027,which agrees with the derivation for revenues. The production capacity as of 2022 was 50,000 lbs, and if we assume linear growth from 2017 to 2022, and from 2022 to 2027, we can impute the capacity for each year and calculate the annual manufacturing recurring cost by multiplying capacity and unit production cost.
Sources:
[2] https://upsidefoods.com/innovation
[3] https://www.ers.usda.gov/data-products/meat-price-spreads/
[4] https://www.zippia.com/memphis-meats-careers-1392392/revenue/
[6] https://www.foxnews.com/leisure/2016/02/03/world-first-lab-grown-meatball-revealed/
[7] https://www.wsj.com/articles/cargill-backs-cell-culture-meat-1503486002
R&D Projects
1. Scaling up operations for mass market (TRL6) - Over 100 start-ups are currently working to mass produce and commercialize affordable lab-grown meat products, which currently exist only as a small-scale and high-cost products available in limited retail contexts.
2. Growth factor-free cell lines (TRL4) - Many companies seek to genetically engineer cell lines to produce their own growth factors, eliminating the need for expensive, additive growth factors.
3. Added scaffold-free cell lines (TRL2) - Similar to growth factors, several companies are beginning to work on genetically engineer cell lines to produce their own scaffold, which reduces the need for added, external scaffolds.
Demonstrator Projects
University of Maastricht burger (2013):
Dr. Mark Post unveiled a five-ounce cultured meat patty created with mulitpotent stem cells (myosatellite cells) as a public-facing proof-of-concept. Post's patty required nearly 20,000 strips of tissue to construct. The $300,000-plus patty received extensive press coverage and tolerant taste reviews. [1,2]
Vow’s Mammoth Meatball (2023):
Using publicly-available DNA data, Vow isolated the gene that coded for myoglobin in woolly mammoths and filled gaps with African elephant DNA. This reconstructed mammoth myoglobin gene was then inserted into muscle cells taken from sheep. Because sheep and mammoths are more distantly related, technicians could easily identify which sheep cells were now producing mammoth myoglobin. The cells expressing mammoth myoglobin were proliferated (to approximately 20 billion cells) before processing into the mammoth meatball which was exhibited at a Dutch science museum. [3]
Sources:
[1] https://www.nytimes.com/2013/08/06/science/a-lab-grown-burger-gets-a-taste-test.html
[3] https://www.theguardian.com/environment/2023/mar/28/meatball-mammoth-created-cultivated-meat-firm
[4] https://www.mammothmeatball.com/the-recipe
Technology Statement and Swoosh Chart
Since lab-grown meat is a new industry with a low TRL of around 4-6, our technology strategy in the near-term future focuses on improving the technology for the production of lab grown meat and scaling it to a level where production can become profitable, and startups can begin selling their products.
Our main strategic drivers are to (1) reduce growth factor costs and the amount of growth medium needed, (2) scale up individual bioreactor capacity to improve output, (3) achieve efficient bioreactor design and techniques for a sustainable production rate. (4) market lab-grown meat at a price competitive with conventional meat.