Difference between revisions of "Messenger RNA Vaccine"
| Line 12: | Line 12: | ||
The discovery process spans from obtaining the genetic information of the target virus to the phase just before the commencement of human clinical trials. | The discovery process spans from obtaining the genetic information of the target virus to the phase just before the commencement of human clinical trials. | ||
[[File:2MRV_03_01.png|frameless| | [[File:2MRV_03_01.png|frameless|3000px|OPM1]] | ||
==Development== | ==Development== | ||
The development process begins with the first human clinical trial and continues until the drug receives approval. | The development process begins with the first human clinical trial and continues until the drug receives approval. | ||
[[File:2MRV_03_02.png|frameless| | [[File:2MRV_03_02.png|frameless|3000px|OPM2]] | ||
==Manufacturing process== | ==Manufacturing process== | ||
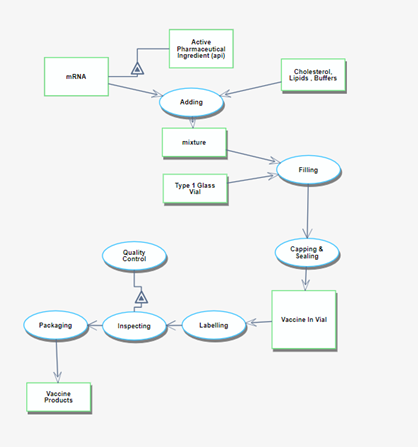
The manufacturing process begins after the drug has been approved. It starts with the assembly of each ingredient component and continues through to the shipment of the finished vaccine products. | The manufacturing process begins after the drug has been approved. It starts with the assembly of each ingredient component and continues through to the shipment of the finished vaccine products. | ||
[[File:2MRV_03_03.png|frameless| | [[File:2MRV_03_03.png|frameless|3000px|OPM3]] | ||
==Actual Vaccination Situation== | ==Actual Vaccination Situation== | ||
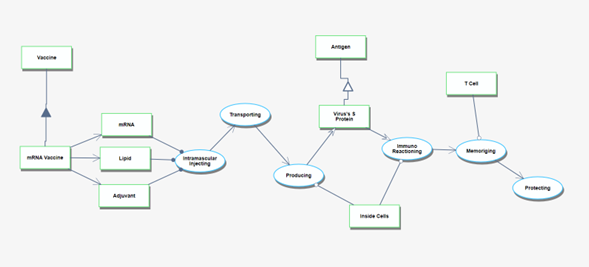
The Actual Vaccination Situation refers to the process that starts with receiving the vaccine products at the vaccination site and culminates in individuals gaining immunity after being administered the vaccines. | The Actual Vaccination Situation refers to the process that starts with receiving the vaccine products at the vaccination site and culminates in individuals gaining immunity after being administered the vaccines. | ||
[[File:2MRV_03_04.png|frameless| | [[File:2MRV_03_04.png|frameless|3000px|OPM4]] | ||
=Figures of Merit= | =Figures of Merit= | ||
Revision as of 23:41, 11 October 2023
Roadmap Overview
XXX
Design Structure Matrix (DSM) Allocation
The DSM illustrates the interplay between the tools and methods that constitute the Messenger RNA Vaccine. A 'X' sign denotes that the techniques are interconnected.
Roadmap Model using OPM
Discovery
The discovery process spans from obtaining the genetic information of the target virus to the phase just before the commencement of human clinical trials.
Development
The development process begins with the first human clinical trial and continues until the drug receives approval.

Manufacturing process
The manufacturing process begins after the drug has been approved. It starts with the assembly of each ingredient component and continues through to the shipment of the finished vaccine products.
Actual Vaccination Situation
The Actual Vaccination Situation refers to the process that starts with receiving the vaccine products at the vaccination site and culminates in individuals gaining immunity after being administered the vaccines.
Figures of Merit
XXX
Alignment with Company Strategic Drivers
XXX
Positioning of Company vs. Competition
XXX
Technical Model
XXX
Financial Model
XXX