Mining Critical Materials from Seawater and Brine
Technology Roadmap Sections and Deliverables
This is a technology roadmap for:
- 4AIX - Adsorption and Ion Exchange
Roadmap Overview
Cobalt is a critical input for lithium-ion batteries, which power everything from laptops and cellphones to electric vehicles to the U.S. power grid. As demand for electric vehicles and other technologies reliant on lithium-ion batteries surges in the coming years, so too will demand for minerals underpinning their supply chains. Cobalt is a critical material used to make the cathode of lithium-ion batteries and some analyses indicate cobalt could become the limiting factor in expansion of technologies powered by lithium-ion batteries as soon as the early 2020’s.<ref>Olivetti, E.A., Ceder, G., Gaustad, G. G., and X. Fu. “Lithium-ion battery supply chain considerations: analysis of potential bottlenecks in critical metals.” Joule 1[2]: 229–243. October 2017.</ref> This is because the majority of global terrestrial cobalt reserves is in the Congo, a politically-fraught region that supplies 70% of global cobalt demand and on which the United States is import dependent.<ref>USGS. “Mineral Commodity Summaries: Cobalt.” January 2020.</ref> Not only does this import-reliance put U.S. consumers at risk of supply disruptions and price spikes, but it also supports a government with a long track record of human rights abuses and environmental degradation.
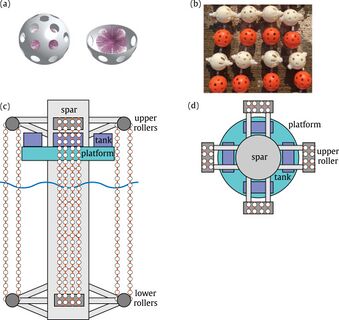
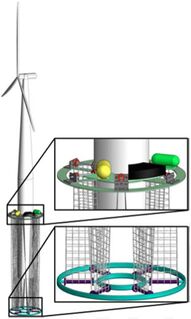
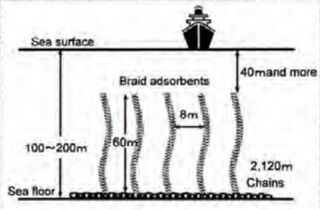
In recent years, chemists have made progress in developing cobalt-free battery cathodes, but many of these either have subpar performance or are at an early stage of development and have not demonstrated commercial proof of concept. Fortunately, there are promising applications for extraction of cobalt and other rare earth minerals from seawater. While the concentration of many minerals in seawater like cobalt is generally low, extraction of both copper and uranium from seawater have proven economically effective when they are attached to existing offshore infrastructure. Below are designs currently being pursued for uranium extraction in which adsorbents are deployed on offshore oil and gas platforms and wind turbines. One recent analysis suggested Design 1 (SMORE) has potential to supply 27.3% of the nation’s 2017 cobalt consumption by retrofitting offshore oil and gas platforms in the Gulf of Mexico.<ref name="S1364032119300838">Haji, M. N. and Slocum, A. H. “An offshore solution to cobalt shortages via adsorption-based harvesting from seawater.” Renewable and Sustainable Energy Reviews 105: 301–309, 2019.</ref> Our roadmap explores the technical, political, and economic feasibility of adsorbent deployment designs for passive extraction of cobalt from seawater.
|
|
|
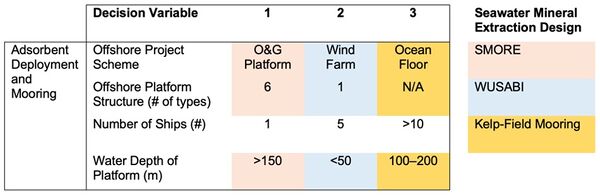
| Design 1: Offshore O&G Platform (SMORE)<ref name="S1364032119300838" /> | Design 2: Offshore Wind Turbine (WUSABI)<ref name="S002980181831713X">Byers, M. F., Haji, M. N., Slocum, A. H., and Schneider, E. “Cost optimization of a symbiotic system to harvest uranium from seawater via an offshore wind turbine.” Ocean Engineering 169: 227–241, 2018.</ref> | Design 3: Kelp Field Mooring<ref>Slocum, Alex. “Extraction of Uranium from seawater: design and testing of a symbiotic system.” Massachusetts Institute of Technology; US Department of Energy. Project No. 14-6557. November 2017. Available at: https://www.osti.gov/biblio/1423067/</ref> |
Design Structure Matrix (DSM) Allocation
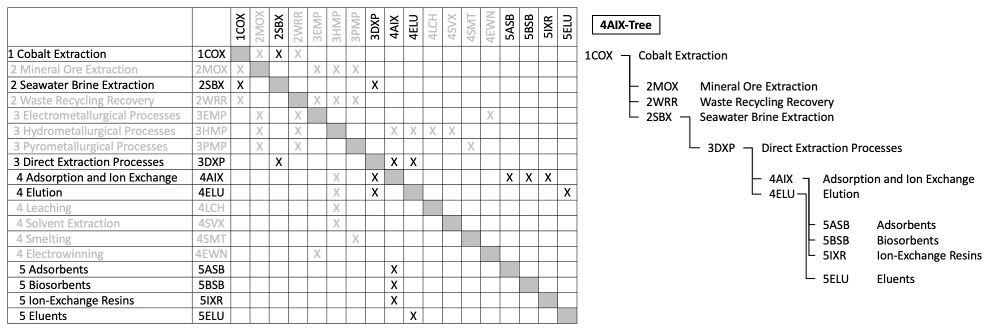
The 4AIX tree extracted from the DSM above shows that seawater brine extraction (2SBX) is a novel method of cobalt extraction (1COX), in comparison to the conventional mineral ore extraction (2MOX) and the emerging waste recycling recovery (2WRR) methods. It requires some enabling technologies in the direct extraction processes (3DXP). Innovation in adsorption and ion exchange (4AIX), as well as elution (4ELU), are some of these promising Level 4 technologies. Within the focus of 4AIX in this roadmap, there is a wide range of promising materials including adsorbents (5ASB), biosorbents (5BSB), and ion-exchange resins (5IXR).
Roadmap Model using OPM
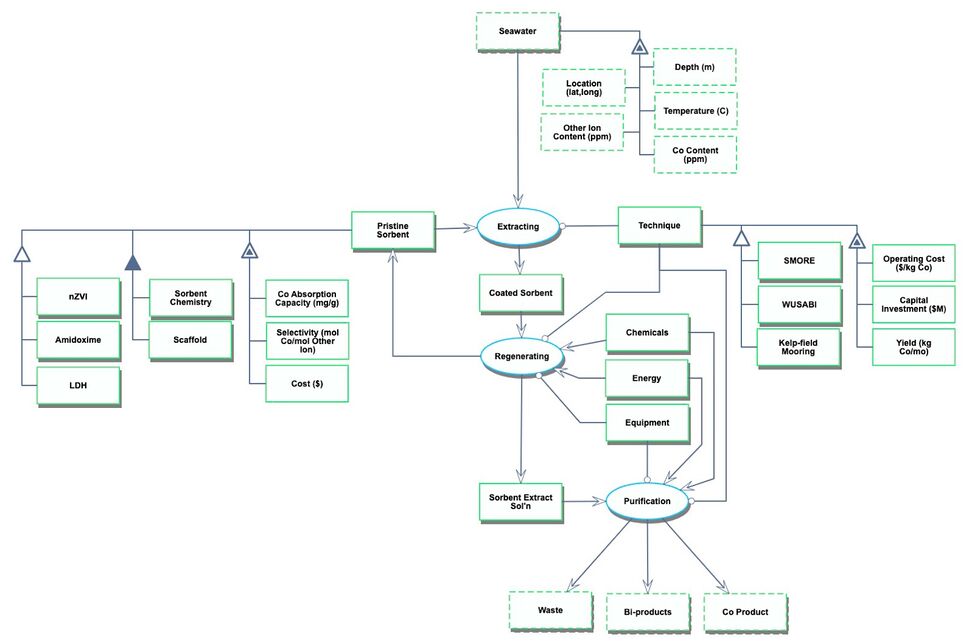
The Object-Process-Diagram (OPD) of the 4AIX roadmap is shown in the figure below. This diagram captures the main object of the roadmap (sorbent), its various instances including nanosized zero-valent iron (nZVI), amidoximes; and its characterization by Figures of Merit (FOMs).
Figures of Merit
| FOM Name | Unit | Description |
|---|---|---|
| Adsorption capacity | [mg/g] |
|
| Selectivity | [dmnl] |
|
| Unit Production Cost | [$/kg] |
|
We gathered reported data from various peer-reviewed publications within the cobalt extraction literature to generate a plot of sorption capacity.
Adsorption capacities of major sorbents.
Alignment with Company Strategic Drivers
Our company provides leading-edge adsorbates within ion-sorption systems submerged in seawater. Currently, we identify the most important FOMs driving strategy as capacity (mg adsorbate/g sorbent) and degradation rate (% reduction in capacity per load/unload cycle). This can be combined into an overall FOM of mg adsorbate/g sorbent over the lifetime of the adsorbate. This is calculated as:
<math display="block">\begin{align}
\text{lifetime capacity}=C_T=\sum_{n=1}^NC{}_i(1-{}r_r)^{n-1}
\end{align}</math>
where <math display="block">\begin{align}n\end{align}</math> is the cycle number, <math display="block">\begin{align}N\end{align}</math> is the total number of cycles, and <math display="block">\begin{align}r_r\end{align}</math> is the degradation rate per cycle, and <math display="block">\begin{align}C_i\end{align}</math> is the initial sorbent capacity.
Below, we provide a set of targets based on a strategic driver involving lifetime capacity (<math display="block">\begin{align}C_T\end{align}</math>).
| Strategic Driver | Alignment and Target |
|---|---|
| To achieve competitive economics with other forms of Co extraction, <math display="block">\begin{align}C_T\end{align}</math> for our technology needs to be 680 mg/g (4x single-cycle nZVI capacity) | Produce a field-deployable adsorbent that can harvest Co for 4 cycles with 0 degradation, or 6 cycles with a constant degradation rate less than that permitted by lifetime capacity equation (7.5%) |
| Infrastructure of Co-harvesting system cannot interfere with sorbent capacity over the course of sorbent lifetime. |
Positioning of Company vs. Competition
See below for a chart outlining the current state of play for several key figures of merit (FOM) for sorbents designed to extract cobalt from seawater. Please note that for some figures of merit where data was either limited or difficult to compile, figures for seawater extraction of uranium were used instead because of their analogous project scopes and figures of merit.
| FOM | Units | Level Achieved | Target |
|---|---|---|---|
| Adsorption Capacity | mg Co/g sorbent | 166 (SiO2-Gly)
172 (nZVI) 196 (Mesosponge) 262 (Hydrogel) |
As high as possible given temperature and pH constraints |
| Selectivity | Dimensionless (dmnl) | High (nZVI) | High |
| Unit Production Cost | $/kg Co | N/A | <USD36.06
(current spot price of cobalt) |
| Reusability | Number of Reuse Cycles | 20 (Uranium) | As many as possible |
| Durability | % retained per re-use | 5% (Uranium) | As high as possible |
Technical Model
Sensitivity Analysis #1: Sorbent Percent Uptake (%)
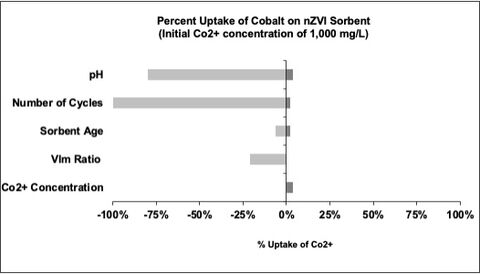
Below is a table listing the parameters we explored in a sensitivity analysis for normalized percent sorbent uptake for nanosized zero-valent iron (nZVI), a highly efficient sorbent that can extract cobalt from seawater with adsorption capacities as high as 172 mg/g. As can be seen in the tornado diagram below, the percent of cobalt adsorbed is highly sensitive to the initial solution pH and the number of times the sorbent is used. Interestingly, adsorption capacity exhibits relatively low sensitivity to the age of the nZVI sorbent, the V/m ratio (aka: the volume of solution per mass of nZVI sorbent), and the initial concentration of cobalt in solution. Other parameters that impact adsorption uptake but are not modeled in our sensitivity analysis (due to limited data availability under the same parameter assumptions) include temperature and contact time with the sorbent.
| Parameters | Description | Units | Range Explored |
|---|---|---|---|
| Co2+ Concentration | Initial Co2+ concentration | mg Co/L | 1 to 1,000 mg/L |
| V/m Ratio | Volume of solution per mass of nZVI sorbent | mL/g sorbent | 50 to 200 mL/g |
| Sampling Age | Time before sample used as adsorbent | days | 0 to 40 days |
| Repetitive Loading | Number of applications of the same nZVI sample | # | 1 to 8 cycles |
| pH | Initial pH | # [1 to 14] | 4 to 10 |
Note: All parameters (except pH) based on 10 mL solution containing 0.2 g nZVI sorben; pH data based on 20 mL solution containing 0.05 g nZVI sorbent. (Source: <ref>Üzüm, Ç., Shahwan, T., Eroğlu, A., Lieberwirth, I., Scott, T., and Hallam, K. (2008). "Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions." Chemical Engineering Journal 144 (2008): 213–220.</ref>)
Sensitivity Analysis #2: Unit Production Cost ($/kg)
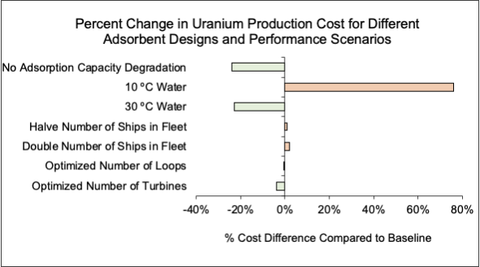
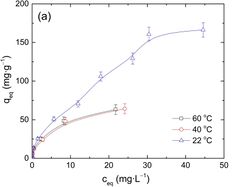
We performed a second sensitivity analysis normalized for percent change in unit production costs of uranium extraction from seawater from adsorbents attached to offshore wind turbines compared to a status quo system in which adsorbents are moored to the ocean floor in a kelp field-like structure. We report the results for uranium because cobalt extraction from seawater is not widely practiced today and limited project cost data is available. However, uranium has a similar concentration in seawater and is economically extracted from seawater so it offers a useful analog by which we can understand cobalt seawater extraction costs and sensitivities. These cost elements are analogous because mooring and deployment costs - which would be used under both uranium and cobalt seawater extraction schemes - are one of the most significant contributors to unit production costs.<ref name="S002980181831713X" />
The production costs underpinning this sensitivity analysis assume the adsorbent system is attached to offshore wind turbines. The sensitivity in production cost to the above parameters may differ if we assume the adsorbents are attached to other offshore structures such as oil and gas platforms, though many of the same parameters will be relevant under a variety of offshore mineral extraction schemes. Below, sorbent capacity degradation and seawater temperature are among the features to which production cost is most sensitive. The degree and magnitude to which cobalt extraction costs are sensitive to the same parameters warrants further investigation.
(Source: <ref name="S002980181831713X" />)
Below is an initial morphological matrix for different seawater mineral extraction designs. We focused this initial morphological matrix on the adsorbent deployment and mooring setup because the technology for our roadmap is very component level, so there aren’t many decision variables aside from the choice of adsorbent. Accordingly, we have gone a level up for our morphological matrix where other key decision variables impact cobalt extraction schematics. Below is a matrix with an initial set of key design variables impacting the efficiency and production cost of cobalt extraction from seawater. Because there is such little data available about seawater extraction of cobalt, much of this data is based on analogous offshore designs for harvesting uranium from seawater. Please note that cells that are unshaded are applicable for all 3 seawater mineral extraction designs.
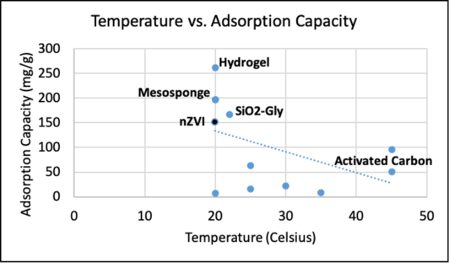
We also developed an initial snapshot of a key element in the tradespace for various sorbents that can extract cobalt from seawater. One parameter that impacts a sorbent’s adsorption capacity is temperature. This is a parameter impactful in the tradespace because seawater temperature varies by location and may impact the first decision variables listed in our morphological matrix - offshore platform scheme. In the United States, much of the offshore oil and gas infrastructure is located in the Gulf of Mexico. By contrast, the only wind farm operating in the United States is off the coast of Rhode Island. The seawater temperature in each of these locations varies drastically and, as such, the tradeoff between adsorption capacity and seawater temperature is important to keep in mind in selecting a cobalt adsorbent. Project developers should select a sorbent towards the upper right corner of the graph below. We will explore further aspects of the tradespace in subsequent iterations of our technology roadmap.
Comparison of adsorption capacity for sorbents at different temperatures.<ref>Piątek, J., Bruin-Dickason, C. N., Jaworski, A., Chen, J., Budnyak, T., & Slabon, A. (2020). Glycine-functionalized silica as sorbent for cobalt(II) and nickel(II) recovery. Applied Surface Science, 530, 147299.</ref>
Financial Model
So far, we have examined potential project schemes for the passive extraction of cobalt from seawater based on analogous designs for seawater uranium extraction that are economically competitive. This makes our financial analysis more straightforward since we have a template to work from, though there are differences we must take into account specific to cobalt. Here, we consider the cost of using sorbents specialized for cobalt vs. uranium extraction, the difference in spot price of each metal, and the absence of ready buyer of cobalt in sufficient quantities (uranium is sold to U.S. breeder reactors).
In our financial analysis, we also assume that the U.S. government offers financial incentives for cobalt extraction from seawater. Such an assumption is optimistic but realistic and is based on historical precedent following the Arab Oil Embargo. The U.S. government has and continues to offer tax credits designed to facilitate advances in and rapidly deploy energy technologies ranging from solar to wind to carbon capture and storage in order to ensure our energy independence. Moreover, ensuring an adequate supply of critical materials that are at risk of supply shocks or price spikes is of national security concern. The United States funds the Critical Materials Institute through the Department of Energy designed to address this risk, and cobalt is included in its purview. As previously discussed, cobalt is a crucial input in lithium ion batteries and the technologies powered by them and the U.S. could be at risk of cobalt shortages as early as the next five years. For these reasons, we felt it was reasonable to assume that the U.S government pays all capital expenses related to retrofitting 40 decommissioned oil rigs ($40 million over 5 years) and that the company can employ depreciation tax benefits on these capital expenditures over 20 years.
Below, we report other assumptions for key parameters underpinning our financial analysis. This analysis is based on the nZVI sorbent which has documented the highest reported adsorption capacity for extracting cobalt from seawater.
| Assumption | Value |
|---|---|
| Operating Margin | 10% (initial)
25% (final) |
| Shells/rig | 11.2k |
| Mass sorbent/shell | 5 kg |
| Discount rate | 15% |
| Cobalt price | $30/kg (initial)
$50/kg (final) |
| Sorbent capacity | 172 g Co/kg sorbent |
| Tax Rate | 21% |
| CapEx of retrofit | $1M/rig |
| Sorption cycle time | 1 month |
| Uptime | 100% |
| Desorption Efficiency | 70% (initial)
90% (final) |
| Fixed Costs (manufacturing, salaries, benefits) | $5M/y |
| Payor for CapEx | Government |
| Tax benefits of depreciation | Provided to sorbent company at linear depreciation |
| Lifetime of rig | 20 years |
| Growth rate for US Co consumption | 2.5%/year |
With these assumptions, we prepared the following discounted cash flow analysis for this project (see below). At a discount rate of 15%, the NPV for this project is $155M, implying that this project will produce value over its lifetime.
Key Publications, Presentations and Patents
Some of the key publications related to cobalt extraction from aqueous solutions:
| Publication | Summary |
|---|---|
| Piątek, J., Bruin-Dickason, C. N., Jaworski, A., Chen, J., Budnyak, T., & Slabon, A. (2020). Glycine-functionalized silica as sorbent for cobalt(II) and nickel(II) recovery. Applied Surface Science, 530, 147299. | Glycine functionalized silica particles (SiO2-Gly) are highly effective sorbents for the removal of Co2+ ions from aqueous solution, with adsorption capacity of 166.3 mg/g. |
| Üzüm, Ç., Shahwan, T., Eroğlu, A., Lieberwirth, I., Scott, T., & Hallam, K. (2008). Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chemical Engineering Journal, 144(2), 213–220. | Examines the uptake of Co2+ ions using nanosized zero-valent iron (nZVI) as an efficient sorbent, with a high uptake capacity of 172 mg/g, reaching equilibrium within 10 minutes at an initial concentration of 10–100 mg/L. nZVI also demonstrates less than 10% loss of reactivity for up to 40 days. |
| Shawabkeh, R., Al-Khashman, O., Al-Omari, H., & Shawabkeh, A. (2007). Cobalt and zinc removal from aqueous solution by chemically treated bentonite. The Environmentalist, 27(3), 357–363. | Demonstrates the enhancement in uptake of Co2+ ions using bentonite treated by HCl, followed by further treatment with NaOH, with an adsorption capacity of 138.1 mg/g predicted by Langmuir model. |
While the Cooperative Patent Classification (CPC) has a subclass C22B, group 23/00 that covers the production of cobalt by a metallurgical process, the patents for the novel extraction of cobalt from seawater are scarce. Therefore, we searched for and found the following related patents of other metals, although most of them have already expired.
| Year | Patent No | Title | Claims Summary | Assignee | Status |
|---|---|---|---|---|---|
| 1986 | US4585627A | Process for the concentration of uranium from sea water | A process for the selective concentration of uranium from sea water through chemical accumulation on a solid adsorption medium, and subsequent elution with carbonate-containing sea water. | Kernforschungsanlage Julich GmbH | Expired |
| 1987 | SU1553538A1 | Cross-linked copolymer of tetravinyl ether of pentaerythritol and maleic anhydride as a sorbent of cobalt from acidic media | An invention that improves the adsorption capacity of cobalt up to 125 mg/g at pH 1–3. | – | Expired |
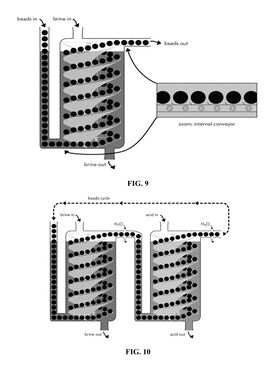
| 2019 | US10439200B2 | Ion exchange system for lithium extraction | A process for the extraction of lithium ions from a liquid source, by using porous beads coated with ion exchange particles. Figures below illustrate the extraction system, which includes columns with a moving bed of ion-exchange beads that move in an opposite direction of liquid flows. | Lilac Solutions Inc | Active |
List of R&T Projects and Prototypes
Given that seawater mining is only at the conceptual stage at present, the list of projects planned will be organized along the Technology Readiness Level (TRL) scale. The strategy is to advance from proof-of-concept from the laboratory to a demonstrator project within 5 years.
- To research adsorbents and related technologies: Further analytical and laboratory studies on selected candidates of adsorbent material, especially to investigate the primary FOMs of adsorption capacity and selectivity in seawater and other conditions (e.g. pH, temperature, contact time).
- To design and prototype the harvesting system: Embodiment of the adsorption and elution processes, designed in accordance with the characteristics of the adsorbent and resulting system requirements.
- To demonstrate the system prototype: Retrofit of an existing offshore structure<ref name="S1364032119300838" />, e.g. a decommissioned oil platform, with the demonstrator system as a testbed for pre-production.
Following production in 2025, R&D will focus on improving the operations as modeled in the NPV analysis, with the following projects:
- Increasing desorption efficiency from 70% to 90% in 4–5 years.
- Improving cost and increasing operating margin from 10% to 25% in 4–5 years, by enabling technologies e.g. faster elution process.
- Enhancing the safety and sustainability in handling cobalt due to its toxicity.
The following Gantt chart illustrates the key milestones:
Technology Strategy Statement
Our target is to develop a passive extraction system of cobalt from seawater with production planned to start in 2024. To achieve the target of meeting over 40% of US cobalt consumption in the next ten years, we will invest in a prototype development project of a harvesting system using nZVI as the adsorbent, which will lead to a demonstrator project and production start within five years, and a scaled-up operation by 2030.
References
<references />