Plasma-based Oxygen Generation in Space
Roadmap Creators: | Lanie McKinney | Roy Weinstock
Technology Roadmap Sections and Deliverables
This technology roadmap has the unique identifier:
2PBOGS - Plasma-based Oxygen Generation in Space
The number 2 denotes that this is a Level 2 technology roadmap at the product level. In reference to our technology, Level 1 encompasses all oxygen generation technologies that can be used in space. Level 3, the system level, references for example the plasma reactor configuration for conversion and Level 4, the subsystem level includes the kind of plasma source utilized.
Roadmap Overview
Oxygen generation in space is a critical enabling technology to support the development of human exploration of the Moon and Mars. NASA's Artemis program plans to head back to the Moon this decade in a phased approach to build up lunar activity, including a commercial and International presence, to serve as a proving ground for human missions to Mars. Increasingly longer stays off-world at destinations of increasing distance from Earth require technologies that recycle and utilize available resources, called in-situ resource utilization (ISRU), to make large mission architectures feasible and enable dramatic cost-savings. Notably, the most near-term large-scale oxygen demand will be driven by lunar lander propellant needs, like Starship and Blue Moon (both contracted by NASA as part of the Human Landing System). One projection estimates that the market valuation of the ISRU sector will reach 63$ billion by 2040, where 99% of the demand is driven by propellant [1]. There are three primary resources available in space to extract oxygen: H2O, CO2, and Regolith. Water and oxygen found in regolith are both available on the Moon and Mars, whereas CO2 is only found in the Mars environment.
Non-thermal plasma reactors are emerging power-to-gas technologies with the potential to facilitate various chemical synthesis processes. In plasma-based systems, electrical power is used to ionize a feedstock gas, creating a highly reactive environment that leverages electron excitation chemistry to break stable molecular bonds and produce value-added products. Plasma reactors operate at non-equilibrium conditions which allows lower-temperature operation and instantaneous start-up, making them adaptable to intermittent power availability. Unlike other technologies, plasma reactors can process any feedstock, and have electrical settings and environmental conditions which can be tuned to optimize or favor particular products. Plasma reactors can reduce regolith and produce water (a source of oxygen) and transform CO2 into oxygen. This means that plasma-based conversion technologies are extensible to both near and far-term missions, and can be used where any of these resources are found on the Moon and Mars.
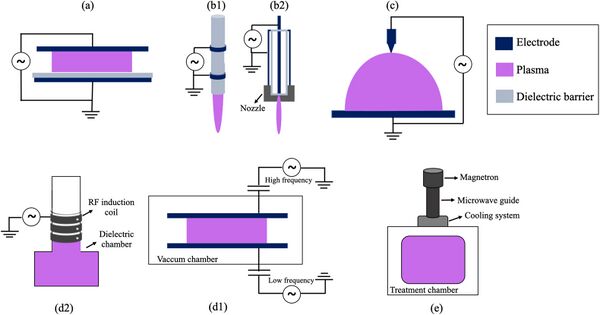
In the diagram above, the plasma-based technologies potential for performing multiple chemical conversion processes relevant to available resources on the Moon and Mars to produce oxygen are shown. In the diagram below, a few of the common plasma discharge reactor configurations are shown, including (a) dielectric barrier discharges, (b1) plasma jet, (c) Corona discharges, (d1) Radiofrequency Inductively coupled plasma, and (d2) AC-driven Capacitively coupled plasmas, and (e) Microwave plasmas [2].
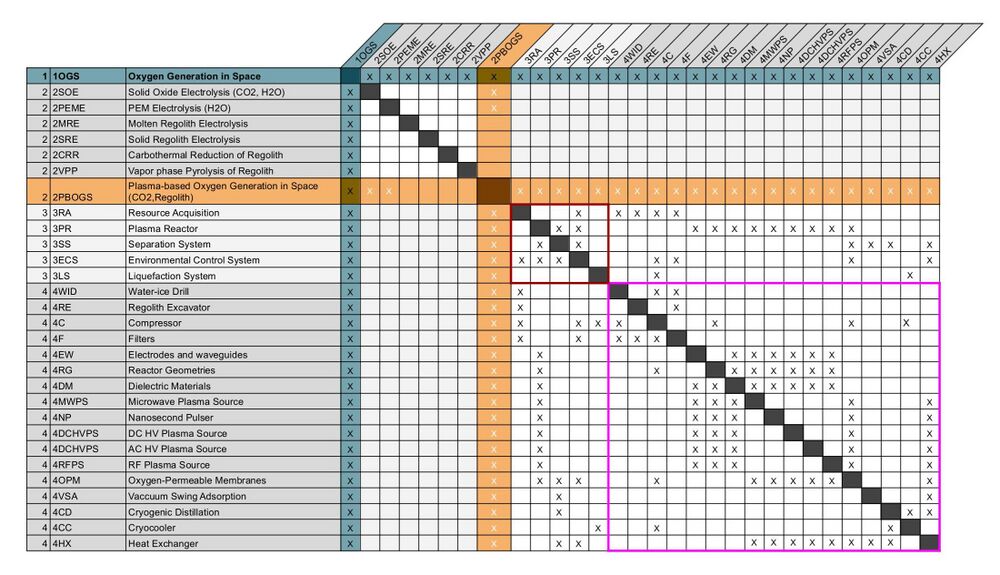
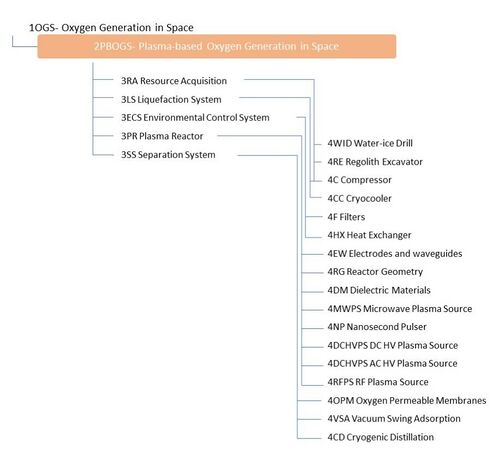
Design Structure Matrix (DSM) Allocation
The market level DSM for Oxygen Generation includes candidate technologies that can extract oxygen from a variety of resources available in space, namely CO2, water, and regolith. It also shows that there can be either a dependency on electrolysis technologies or opportunities to combine them with plasma methods for enhanced performance. At the system level, key enabling technologies are the resource acquisition system (3RA), the plasma reactor (3PR), separation system (3SS), the environmental control system (3ECS), and the liquefaction system (3LS). At level 4, the enabling technologies include the acquisition systems needed to extract the different resources (4C, 4WID, 4RE), the systems to control the conditions of the input stream (4F, 4HX, 4C), 5 different kinds of plasma sources (3MWPS, 3NP, 3DCHVPS, 3ACHVPS, 3RFPS) which generate plasmas in different ways, 3 technologies related to reactor design (4RG, 4DM, 4EW), 3 relevant kinds of separation technologies (3OPM, 3VSA, 3CD), and subsystems needed to facilitate liquefaction (4CC, 4C). The DSM at this level demonstrates the cluster of interdependencies around the selection of the the plasma source and elements of the overall reactor configuration. Different combinations of level 4 elements, like the plasma source, reactor geometry, and separation subsystem may lead to novel performance.
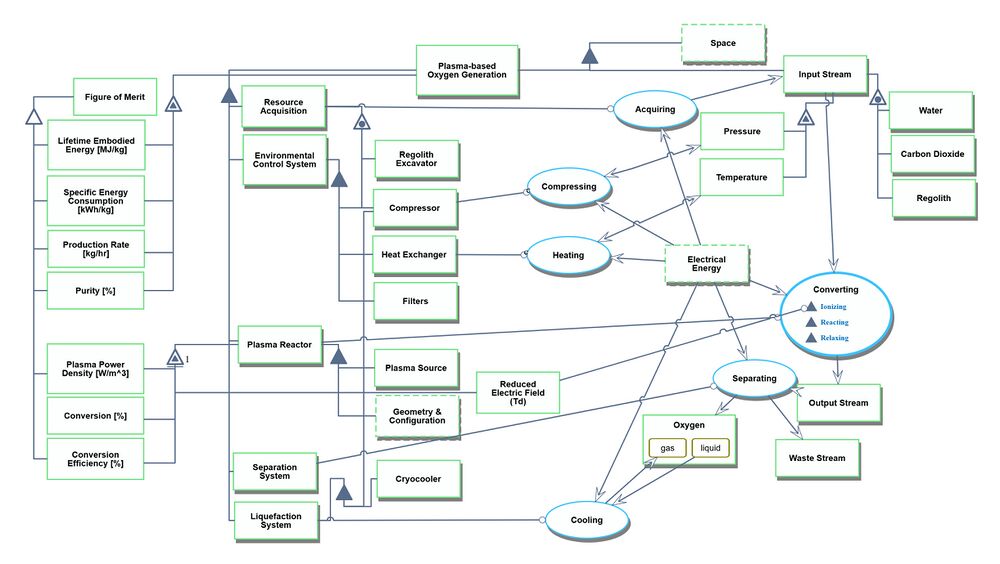
Roadmap Model using OPM
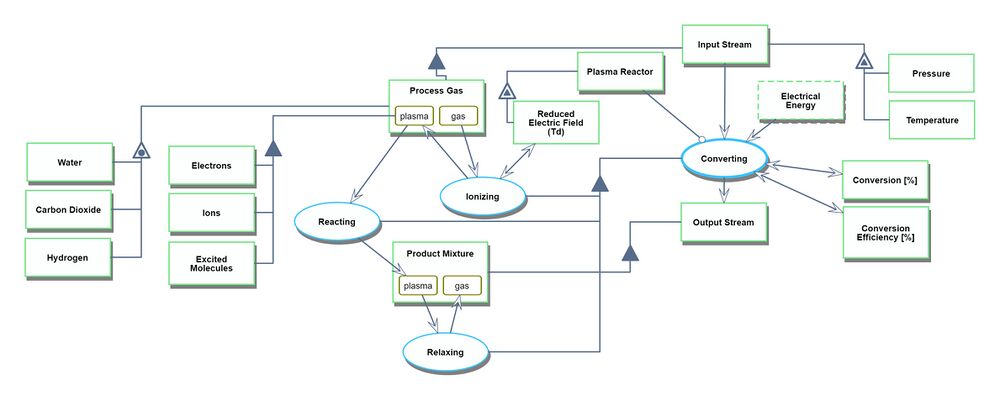
An Object-Process-Diagram (OPD) of the 2PBOGS roadmap is given in the figure below. This diagram captures the main product of the roadmap (level 2) and decomposes the product into four main systems (resource acquisition, environmental control, plasma reactor, and separation) corresponding to level 3 in the DSM and displays the figures of merit (FOMs).
Unfolding the converting process at level SD1
An Object-Process-Language (OPL) description of the roadmap scope is auto-generated and given below. It reflects the same content as the previous figure, but in a formal natural language.
Figures of Merit
The table below show a list of FOMs by which Plasma-based Oxygen Generation technologies can be assessed. The first four (shown in bold) are used to assess the technology compared to other competing technologies in level 2, enabling comparisons at the market level. The following rows represent subordinated FOMs which impact the performance and true cost of this technology, but are provided as outputs (primary FOMs) from lower level roadmaps at level 3 or level 4, see the DSM above. Additionally, the last three FOMs are captured in the first-four composite metrics.
| Figure of Merit | Unit | Description |
|---|---|---|
| Lifetime Embodied Energy | MJ/kg | the thermodynamic sum of past, present and future work required to create, operate, maintain and decommission a system |
| Specific Energy Consumption | kWh/kg | total energy required to produce a kg of product, a measure of energy efficiency |
| Production Rate | kg/hr | resource mass generation rate which captures the benefit any ISRU technology (can be specified as either average or maximum) |
| Purity | % | percentage of the target product in separated product stream, measures the performance of the conversion-separation systems |
| Plasma Power Density | W/m3 | the power delivered to the Plasma normalized by the gas density, which drives chemical reactions |
| Conversion | % | ratio of useful product generated to the total available input reactant |
| Conversion Efficiency | % | ratio of theoretical energy required for targeted chemical conversion to the total energy consumed by the process |
| Total System Power | kW | the total power required to operate the complete system |
| Total System Mass | kg | total mass of all subsystems |
| System lifetime | hr | the total length of time system can operate until end-of-life |
Important FOMs (bolded) such as Lifetime Embodied Energy and Specific Energy Consumption can be calculated from the equations in the table below, though there are dependencies on other major and subordinate FOMs (plain text) like conversion, conversion efficiency, etc which must be obtained through simulations, experiments or a combination of both. Inputs that are indented and italicized are environmental and reactor variables and conditions.
| Inputs | Key Relationships/Equations | Outputs |
|---|---|---|
| Reduced Electric Field E⁄n Gas density Electron density |
Pplasma = e μe ne n2 ( E⁄n )2 = f( E⁄n ) ne n |
Plasma Power Density |
| Plasma Power Density Reactor Temperature Reactor Pressure Flow rate |
simulations - experiments | Production Rate Purity Conversion |
| Plasma Power Density Conversion (α) Reaction Enthalpy (kJ/mol) Molar Flow rate Gas Density |
ηconv = ΔH α n⁄vflow Pplasma | Conversion Efficiency |
| Production Rate Total System Power |
SEC = Ptot⁄rprod | Specific Energy Consumption |
| Specific Energy Consumption Production Rate System Lifetime Total System Mass Δ v |
LEE = 3.6 × SEC + 1⁄2 (η mtot (Δv)2)⁄ (t rprod 106) | Lifetime Embodied Energy |
Notes
Plasma power density, although a subordinate FOM, is a key governing equation which demonstrates the fundamental mechanism which drives the chemical reactions. The equation implies the important dependence on the reduced electric field, where different plasma sources and generation mechanisms obtain different ranges of E/n, and this greatly influences the chemistry inside the reactor.
Lifetime Embodied Energy (LEE) is defined as “...the thermodynamic sum of past, present and future work required to create, operate, maintain and decommission a system, including appropriate shares of indirect contributions from upstream systems as well as from other systems in a system-of-systems [3].” This metric has been shown to reproduce results from Equivalent System Mass and IMLEO analyses while decoupling from launch mass (which is becoming an outdated cost proxy). This FOM utilizes the total energy required across the whole architecture's lifetime as a proxy for cost, and normalizes it with the lifetime mass produced. This (cost/benefit) is an extremely relevant metric to the space industry for determining what the real cost of developing infrastructure and operating a space resource generation technology over long time periods to capture economies of scale and identify promising business use cases.
The conversion efficiency reported, a commonly used metric from the plasma community, is not suitable for comparison to "faradaic efficiency" which is used by the electrolysis community. This is a huge problem in the literature, where different technological communities and disciplines report different kinds of efficiency metrics which may obscure the fundamental limits or benefits of the technology in comparison to others. A more general FOM, like SEC, should be used for all comparisons across different technologies.
Time Evolution of Figures of Merit
Both ISRU technologies and plasma-based chemical conversion technologies are in the early stages of development, and no full-scale product exists using the technology at this time. However, detailed study of plasma reactors for CO2 conversion at level 3 has led to a plethora of literature, detailed modeling and experiments, databases classifying results, and enhanced understanding of the critical technology at the roadmap level 3PR (see the DSM above). Identified FOMs like conversion and conversion efficiency can be modeled overtime to assess the progression of this technology.
Like with many technologies, there is a trade-off between FOMs. Driving conversion higher in plasma reactors typically requires increasing power deposition which worsens energy efficiency, and vice versa. This can be visualized by the pareto frontier in this reference image.
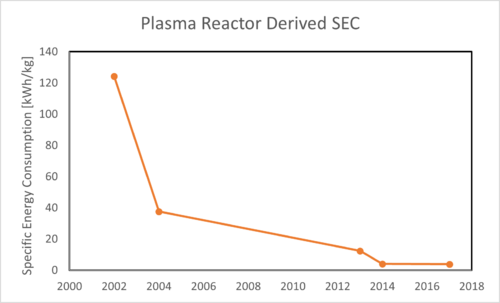
Specific energy consumption is a more general FOM for comparison to other technologies, and it generally trends down with increases in energy efficiency, seen in the plot below. In each of these cases, only plasma power is taken into account, so they are all underestimates of the SEC of a full system, though the conversion system will likely drive the power requirements.
- There are experiments from the 70s and 80s that predate some of the data tracked in conversion and efficiency, however much of that experimental data was not reproduced in the ranges achieved until recently. All data obtained and processed for further analysis in these plots was obtained from the Pioneer Database [4].
Alignment with company strategic drivers
Our “company” is a space resources start-up aiming to develop a common plasma-based oxygen generation technology architecture which can support the anticipated propellant demand on the Moon and Mars. This architecture, much like Starship's original and Lunar variants, will utilize the same underlying technology and modify the system for the two environments to enable rapid and cost-efficient development. The table below shows the three main strategic drivers and the necessary alignment of the 2PBGOS technology roadmap with them.
| Strategic Drivers | Alignment and Targets |
|---|---|
| To explore and assess novel plasma reactor configurations to search for synergistic and optimized performance by 2028. | The 2PBGOS roadmap is targeting the demonstration of reactor prototypes which can reduce demonstrated Specific Energy Consumption by ~22 kWh/kg for both CO2 conversion and regolith reduction (Mars and Lunar variant). |
| To design and develop a full system architecture, including a Lunar and Mars variant, capable of producing a high-purity stream of oxygen from available local resources by 2035. | The 2PBGOS roadmap will target a plasma-based oxygen generation system which can achieve an SEC of 25/8 kWh/kg, a production rate of 1/3 kg/hr, a gas stream purity of 99.6%, and an LEE of 30/80 MJ/kg for the Lunar and Mars variant respectively. |
| To develop a plasma-based system which can directly generate separate streams of oxygen and hydrocarbons, like CH4 and C2H4, from a stream of CO2 and water by 2040. | The 2PBGOS roadmap promotes investment in technology development which can be directly extended to this driver. Although related, this roadmap is currently not aligned with this driver. |
The list of drivers shows that the company views plasma-based oxygen generation as a potential new fuel production business in space and wants to develop it as a commercially viable (for profit) business (1). In order to do so, the first and second drivers aim to match or exceed demonstrated and expected performance of MOXIE's larger-scale counterpart BAM to offer competitively priced propellant on Mars, as well as relieve the requirement of mining water in lunar PSR's while offering competitively priced oxygen on the Moon compared to other regolith ISRU methods [5]. The third driver is not aligned with this roadmap, although this roadmap does strategically invest in enabling technologies and knowledge acquisition and identified relevant, high-level FOMs like LEE and SEC which directly apply to this technology. This technology would simultaneously produce oxygen and hydrocarbons, extending and branching from the focus of this roadmap. This driver is notable because in addition to use on Mars, this technology has the potential to tap into Earth-based markets for sustainable reforming of CO2 and H2O into value-added products.
Positioning of company vs. competition
The table below shows a summary of FOM for other oxygen-generation technologies obtained from public data, where the first three reported FOM values are what has been achieved to date. Predicted SEC and LEE of the full system, as well as TRL designations are included to holistically evaluate the progression of these technologies, recognizing that many of them will have enhanced performance when scaled up beyond the demonstrator stage and that the true cost of utilizing the technology will only be fully understood at scale.
| Oxygen Generation Technology | Resource | Product/ Project Name | Company/Agency | SEC (kWh/kg) | Production Rate (kg/hr) | Purity (%) | Predicted SEC (kWh/kg) | Predicted LEE (MJ/kg) | TRL | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Solid Oxide Electrolysis | CO2 | MOXIE | OXEON, NASA | 31 | 0.01 | >99.6% | 8.98 | 33.9 | 6 | [4] |
| PEM Electrolysis | H2O | IHOP | Paragon/Griner | 5.83 | 0.65 | >99% | 11.425 | 21.1 | 4 | [6] |
| Molten Salt Electrolysis | Regolith | ROXY | Airbus | - | 0.000143 | - | 16.4 | 64.3 | 3 | [7] |
| Molten Regolith Electrolysis | Regolith | - | MIT, NASA | - | - | - | 23.8 | 86.0 | 4 | [8] |
| Carbothermal Reduction | Regolith | CARD | Sierra Space | 45.7 | - | - | 42.7 | 157.3 | 5 | [9] |
The following companies have recently indicated they are developing oxygen production technologies, sometimes even receiving development funds in the form of a government contract, but no performance information is available at this time: Regolithix, Helios, Blue Origin, Lunar Resources, Ethos space, Cislune, iSpace.
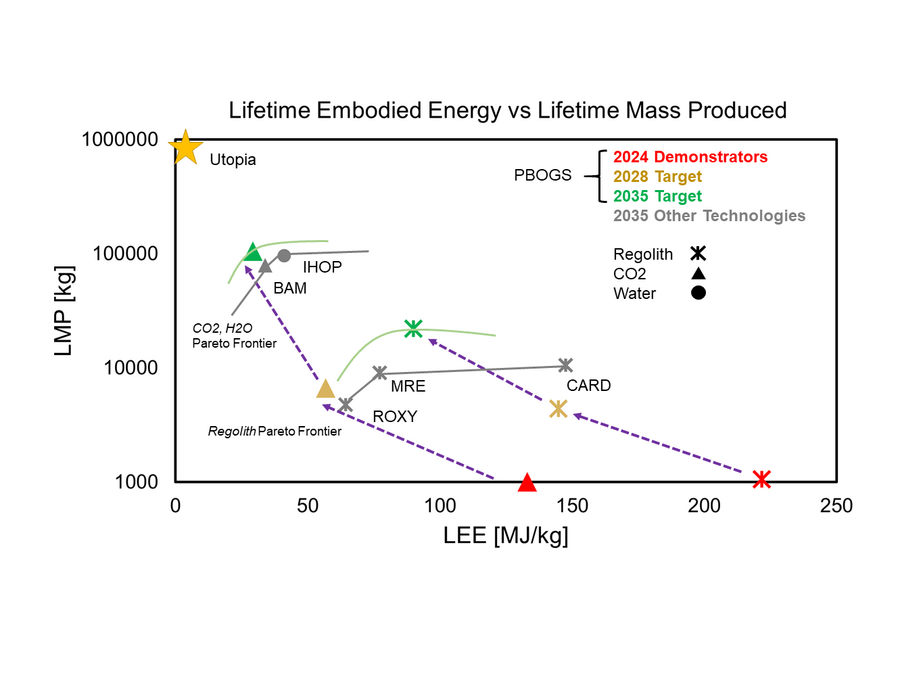
In many cases above, there is not enough current information about the laboratory scale developments taking place to place all technologies in their current context, although many papers include projected metrics based on detailed design studies of scaled-up systems. While these FOMs may be optimistic, they can begin to communicate how these different technologies compare at full-scale implementation to drive investment strategies. Notably, the demonstrator stages focus on the reported performance of the ISRU conversion subsystem. But for estimates of the scaled-up plant, estimates of the oxygen liquefaction and resource extraction energy (like water mining in PSRs [10]) are included in the final estimated values shown in the chart below. Although electrolysis systems are used on Earth, space-based systems have yet to reach the market, so the pilot plant estimates of competing technologies include all technological advancements until the target year of 2035, when these technologies are expected to enter the market.
As you can see, the projected performance of MOXIE (BAM) and IHOP inhabit the current estimated pareto frontier for ISRU oxygen generation, which cover ISRU on both the Moon and Mars. MOXIE is also the most advanced ISRU technology to date, because it is the technology demonstrated on another planetary body. However, the O2 propellant value chain begins in the near-term at the Moon, and CO2 is not an available resource. The options then are to electrolyze water mined from Lunar permanently shadowed regions as the IHOP architecture requires, or extract it from regolith. The value proposition of extracting oxygen from regolith is twofold: it relaxes the requirement of producing propellant near water, and metal byproducts of the process begin the start of it's own Lunar value chain (not quantified in this roadmap). However regolith processing has proven to be more challenging and energy-intensive, seen in the "regolith" pareto frontier lagging behind other technologies in terms of pure oxygen production.
Target 1 aims to take each variant from a TRL 3 proof-of-concept stage to a TRL 4 breadboard reactor prototype which includes investigating different reactor configurations for optimized performance. Because these two variants have only been recently established as TRL 3, the projected jump in performance is reasonable if given appropriate R&D investment. In the case of CO2, target 1 matches the performance of the plasma subsystem at Level 3 of the DSM, but also requires the incorporation and improvement on the separation subsystem. In the case of regolith reduction, no molten materials or high-temperatures are required for the improvement of its performance. Target 2, which deals with the architecture level, aims to develop a plasma system with two variants for the Moon and Mars and respectively. The regolith reduction variant aims to push the regolith pareto frontier forward by eliminating the challenging engineering requirements of handling high-temperatures, molten materials, or multiple chemical processes and combining with the state-of-the-art water-electrolysis technology. Target 3 concerns the CO2 variant and the Mars environment specifically, with an aim to produce oxygen and methane simultaneously in a single-step reactor, producing all the propellant required to fuel large LCH4/LOX engines, though this investment is not aligned with the current roadmap and will extend into the 2040s when missions to Mars may be more frequent. Target 1 is a demonstrator milestone for both variants and Target 2 is a realistic goal given the architecture-level FOMs on an ambitious timeline to meet the propellant demand needs at the Moon and Mars.
Technical model: Morphological matrix and tradespace
In order to assess the feasibility of technical targets at the level of the 2PBGOS roadmap, it is necessary to develop a technical model. There are three major discrete design variables that must be considered in the reactor design, also captured in the dependencies in the DSM, and the table below shows these choices.
| Key Architecture Decisions | Affects | Options | ||||
|---|---|---|---|---|---|---|
| Plasma Source | E/n, Plasma Power, Lifetime, Conversion/Efficiency, System Mass | HV DC | HV AC | Nanosecond Pulser | MW | RF |
| Geometry | Conversion/Efficiency, System Mass | Plate-to-Plate | Coaxial | Standard GA | GA Plasmatron | MW or RF |
| Separation System | Total Power, Purity | Cryogenic Distillation | Vacuum Swing Adsorption | Mixed Ionic Electronic Conducting Membranes | Solid Oxide Electrolysis Separation Membrane |
Other key decisions are reactor operating temperature, pressure, and flow rate where optimal configurations require detailed chemical kinetic and physics-based simulations and are the subject of a level 3 plasma reactor roadmap. To develop a technical model at the level of this roadmap, the best-available data captured for CO2 conversion will be used to place the full system in context and perform a sensitivity analysis on FOMs SEC and LEE, which are related to but somewhat disjointed from the morphological matrix.
A design vector formulated using a series of reference values for the current state of the art of CO2 conversion for Mars ISRU (and scaled up x100) provided in [11] is presented below. A linear scaling law was implemented, as it is established that in current configurations plasma reactors power tends to scale linearly with production rate [].
| Plasma Source & Geometry | Separation System | Temperature | Pressure | Plasma Power | Total Power | Production Rate | System Mass | System Lifetime | SEC | LEE |
|---|---|---|---|---|---|---|---|---|---|---|
| MW | SOEC | 250 K (Mars Ambient) | 5 Torr (Mars Ambient) | 20 kW | 30 kW | 1.4 kg/hr | 600 kg | 100,000 hrs | 21.4 kWh/kg O2 | 77.2 MJ/kg |
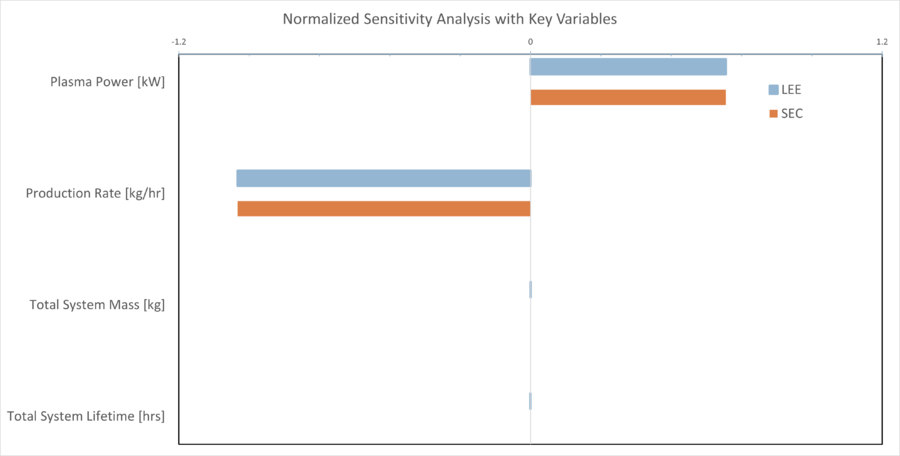
Key insights from this plot are that SEC drives LEE, and subsequently the effect of mass and system lifetime are negligible in comparison. This LEE analysis is not taking into account any modifications, maintenance, or in-situ fabrication of parts. The key underlying challenge is to find power savings, improve the efficiency of the conversion process, or find configurations that enable optimal power scaling with production rate. Additionally, in this configuration Plasma power is the primary contribution to the total power budget, although the separation system makes up the second largest-contribution, and coupled plasma conversion-separation systems are still an open area of investigation. This demonstrates alignment with Target 1 and provides focus and direction for building a prototype reactor.
Keys Patents and Publications
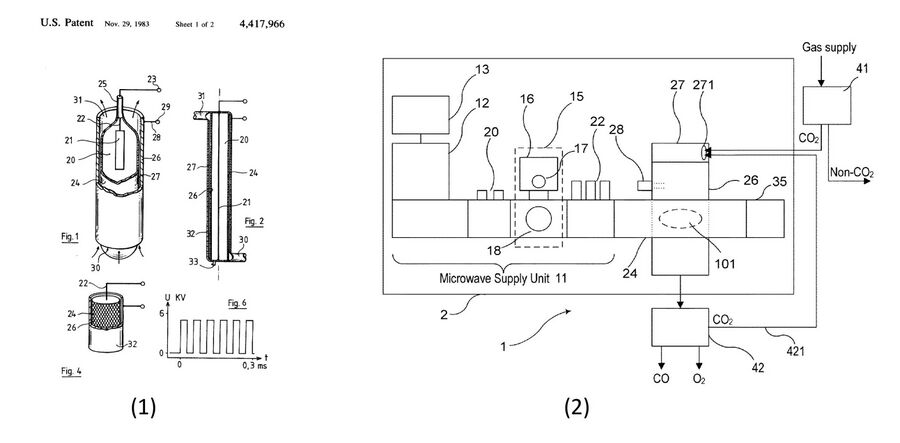
A patent analysis was performed. A challenge with patent analyses using the US patent database is that the term "plasma" also can refer to blood plasma, so many unrelated medical patents will appear in the search. The most effective patent analysis tool for this technology was Google Scholar, using the word "patent" in the search keywords along with phrases like "plasma chemical conversion" and "non-thermal plasma reactor". The search yielded patents for many different reactor configurations, where reactor design has been modified (sometimes only in very small ways) compared to others for enhanced production in some particular process. Two key patents have been identified which have been commercially realized. The first patent, "Apparatus and method of producing ozone" awarded to Innovatron Krauss & Co. in 1981, is the earliest example of plasma-based chemical conversion device on an industrial scale [12]. This device uses one of the most well studied reactor configurations to date, an AC dielectric barrier discharge, to generate ozone (O3) from input oxygen (O2). The patent has since expired, but the device has inspired the use of the reactor to study many kinds of chemical conversion processes, including CO2 conversion, water splitting, the reforming of methane, ammonia synthesis, sustainable aviation fuel synthesis, and more. The second patent, "Gas Conversion System" awarded to Silicon Valley-based company Recarbon Inc. in 2014, is a general, MW plasma reactor designed for scalability and commercial implementation [13]. The system specifies CO2 conversion but can be utilizes with any process gas. In fact, the company currently utilizes this system for the dry reforming of methane (CO2+CH4) to produce negative-carbon value-added products.
Key publications were analyzed, including two which discuss the applications of plasma-based oxygen production from available resources in both the Lunar and Mars environment. The first publication discusses the benefits of utilizing plasma-based CO2 conversion (among other plasma-based technologies) on Mars, summarizing the current-state of the art in CO2 conversion research, and the next steps toward developing this technology for application in space. Based on current performance of small plasma reactors which is still at the laboratory scale, it is estimated that a specific energy consumption of ~21 kWh/kg are feasible at the system level, which is competitive with MOXIE and can be further optimized [14]. The second publication, a summary of several plasma efforts being studied at NASA Kennedy Space Center, discusses the advantages of using plasma-based regolith reduction compared to other methods, which include avoiding molten products and heating a reducing agent while still creating a reactive environment. This publication also discusses their proof-of-concept demonstration and provides their experimental results in terms of water yield, though FOM estimates were not provided and warrant further investigation [15].
In summary, most of the relevant patents focus on a particular reactor configuration for a specified process, with no patents emerging for plasma-ISRU-specific systems, though the exploration of many different processes for these different reactor configurations have been carried out in the literature. The key publications identified focus on the feasibility of these technologies for ISRU and their competitiveness compared to other available technologies
Bibliography
[1] Scatteia, L., & Perrot, Y. (2021). Lunar market assessment: Market trends and challenges in the development of a lunar economy. PwC. Prepared by PwC Space Practice. https://www.pwc.fr/space.
[2] Laroque, D. A., Seó, S. T., Ayala Valencia, G., Laurindo, J. B., and Carciofi, B. A. M., “Cold plasma in food processing: Design, mechanisms, and application,” Journal of Food Engineering, Vol. 312, 2022, p. 110748. https://doi.org/10.1016/j.jfoodeng.2021.110748
[2] Lordos, G. C., Hoffman, J. A., and Summers, S. E., “Towards a Sustainable Industrial Development of Mars: Comparing Novel ISRU / ISM Architectures Using Lifetime Embodied Energy,” 2018 AIAA SPACE and Astronautics Forum and Exposition, 2018. https://doi.org/10.2514/6.2018-5125
[3] Hoffman, J. A., Hecht, M. H., Rapp, D., Hartvigsen, J. J., SooHoo, J. G., Aboobaker, A. M., McClean, J. B., Liu, A. M., Hinterman, E. D., Nasr, M., Hariharan, S., Horn, K. J., Meyen, F. E., Okkels, H., Steen, P., Elangovan, S., Graves, C. R., Khopkar, P., Madsen, M. B., Voecks, G. E., Smith, P. H., Skafte, T. L., Araghi, K. R., and Eisenman, D. J., “Mars Oxygen ISRU Experiment (MOXIE)—Preparing for Human Mars Exploration,” American Association for the Advancement of Science, Vol. 8, No. 35, 2022, p. eabp8636. https://doi.org/10.1126/sciadv.abp8636
[4] Hoffman, J. A., Hinterman, E. R., Hecht, M. H., Rapp, D., & Hartvigsen, J. J. (2022). 18 months of MOXIE (Mars Oxygen ISRU Experiment) operations on the surface of Mars - Preparing for human Mars exploration. In Proceedings of the 73rd International Astronautical Congress (IAC) (IAC-22, A5.2, x72879). International Astronautical Federation.
[5] Seidel, A., Altenburg, M., Monchieri, E., Strigl, F., Quadbeck, P., Redlich, C., & Pal, U. (2022). ROXY - An economically viable process to produce oxygen and metals from regolith. In 51st International Conference on Environmental Systems, 10-14 July 2022, St. Paul, Minnesota. Airbus Defence and Space, Claude Dornier Straße, 88090 Immenstaad, Germany; Fraunhofer Institute for Manufacturing Technology and Advanced Materials IFAM, Winterbergstraße 28, 01277 Dresden, Germany; Boston University, 110 Cummington Avenue, Boston, MA 02215, United States. https://ttu-ir.tdl.org/items/b0da04ae-9619-46b1-8c03-98691c12d252
[6] Holquist, J. B., Joyce, C., Rivera, R., Tewes, P., Myles, T., Markham, D., Ebaugh, T., Rich, M., & Willey, J. (2023). Demonstration of Paragon’s ISRU Propellant Production Subsystem Electrolyzer and Electrolysis Assembly. In 52nd International Conference on Environmental Systems (ICES-2023-108), 16-20 July 2023, Calgary, Canada. Paragon Space Development Corporation, Tucson, AZ, USA; Giner, Inc., Auburndale, MA, USA; and Plug Power, Concord, MA, USA. https://ttu-ir.tdl.org/items/9c946789-6757-417a-a4df-b0208c60548f/full
[7] White, B. C., & Haggerty, N. P. (2023). Carbothermal Reduction System Overview and Developments in Support of the Artemis Program and a Commercial Lunar Economy. In 52nd International Conference on Environmental Systems, 16-20 July 2023, Calgary, Canada. Sierra Space, Madison, WI, USA. https://ttu-ir.tdl.org/items/3731a7a5-b068-4d33-9d40-ee788e57a3a1
[]
[9] Csank, J. (2023). NASA Lunar Surface Operations & Power Grid. CURENT Industry Days, Center for Ultra-Wide-Area Resilient Electric Energy Transmission Networks, University of Tennessee, Knoxville, TN, April 18-19.
[5] Krauss, R., & Koehne, R. (1983). Apparatus and method of producing ozone. U.S. Patent No. 4,417,966. U.S. Patent and Trademark Office. https://patents.google.com/patent/US4417966A
[6] Tanibata, T., Koo, J.-M., & Lee, S. H. (2014). Gas conversion system. U.S. Patent No. 8,633,648. U.S. Patent and Trademark Office. https://patents.google.com/patent/US8633648B2
[7] Guerra, V., Silva, T., Pinhão, N., Guaitella, O., Guerra-Garcia, C., Peeters, F. J. J., Tsampas, M. N., & van de Sanden, M. C. M. (2022). Plasmas for in situ resource utilization on Mars: Fuels, life support, and agriculture. Journal of Applied Physics, 132(7), 070902. https://doi.org/10.1063/5.0098011
[8] Engeling, K. W., & Gott, R. P. (2023). Plasma for crewed transit and planetary habitation: Research efforts at Kennedy Space Center. IEEE Transactions on Plasma Science, 51(6), 1568-1579. https://doi.org/10.1109/TPS.2023.3277216
[9] Rehman, F., Abdul Majeed, W. S., & Zimmerman, W. B. (2013). Hydrogen production from water vapor plasmolysis using a DBD-corona hybrid reactor. Energy & Fuels, 27(5), 2748-2761. https://doi.org/10.1021/ef301981f